11th Standard Chemistry Subject Question Paper Software Subscription
QB365 covers complete information about Tamilnadu 11th Standard 2024-2025 Chemistry Subject. Question Bank includes 11th Standard 2024-2025 ChemistrySubject Book back questions, other important questions, Creative questions, Extra questions, PTA questions, Previous Year asked questions and other key points also. All question with detailed answers are readily available for preparing Chemistry question papers.
All Chapters Covered

Create Unlimited Question Papers

Access anywhere anytime

Multiple Pattern Question Papers
Share your Question Paper

Font size, line spacing, watermark etc,
Other Subjects for Tamil Nadu State Board 11th Standard
11th Standard Chemistry English Medium Chapters / Lessons 2024-2025
Basic Concepts of Chemistry and Chemical Calculations
Quantum Mechanical Model of Atom
Periodic Classification of Elements
Hydrogen
Alkali and Alkaline Earth Metals
Gaseous State
Thermodynamics
Physical and Chemical Equilibrium
Solutions
Chemical bonding
Fundamentals of Organic Chemistry
Basic Concept of Organic Reactions
Hydrocarbons
Haloalkanes and Haloarenes
Environmental Chemistry
11th Standard Chemistry English Medium Chapters / Lessons 2024-2025 Syllabus
Basic Concepts of Chemistry and Chemical Calculations
Chemistry-The Centre of Life-Classification of Matter-Atomic and Molecular Masses-Mole Concept-Gram Equivalent Concept-Empirical Formula and Molecular Formula-Stoichiometry-Redox Reactions
Quantum Mechanical Model of Atom
Introduction to Atom Models-Wave Particle Duality of Matter-Heisenberg’s Uncertainty Principle-Quantum Mechanical Model of Atom – Schrodinger Equation-Quantum Numbers-Filling of Orbitals
Periodic Classification of Elements
Introduction-Classification of Elements-Moseley's Work and Modern Periodic Law-Nomenclature of Elements with Atomic Number Greater Than 100-Grouping of Elements Based on Electronic Configurations-Periodic Trends in Properties-Periodic Trends in Chemical Properties
Hydrogen
Introduction-Preparation of Hydrogen-Properties of Hydrogen-Uses of Hydrogen-Compounds of Hydrogen-Heavy Water-Hydrogen Peroxide-Hydrides-Hydrogen Bonding
Alkali and Alkaline Earth Metals
s-block Elements-Alkali Metals-General Characteristics of the Compounds of Alkali Metals-Biological Importance of Sodium and Potassium-Alkaline Earth Metals-General Characteristics of the Compounds of the Alkaline Earth Metals-Biological Importance of Magnesium and Calcium
Gaseous State
Introduction-The Gas Laws-Ideal Gas Equation-Mixture of Gases-Dalton’s Law of Partial Pressures-Deviation from Ideal Gas Behaviour-Pressure-Volume Isotherms of Carbon Dioxide-Liquefaction of Gases
Thermodynamics
Introduction-System and Surrounding-Zeroth Law of Thermodynamics-First Law of Thermodynamics-Enthalpy (H)-Thermochemical Equations-Measurement of ΔU and ΔH Using Calorimetry-Hess’s Law of Constant Heat Summation-Lattice Energy (ΔHlattice)-Second Law of Thermodynamics-Gibbs Free Energy (G)-Third Law of Thermodynamics
Features in Question Paper Preparation software

(or) type Question

Add or Remover

Sub Questions
Adding Notes
Multiple Pattern

All subjects available
How to Create 11th Standard Chemistry English Medium Question Paper
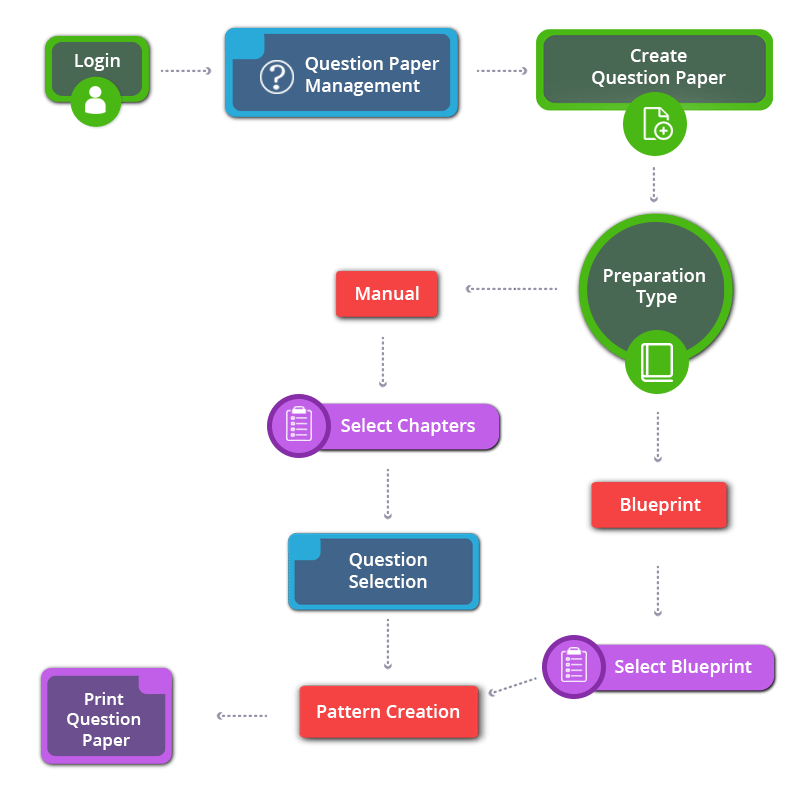

11th Standard Chemistry English Medium Chapters / Lessons 2024-2025
- Covers all chapters
- Unique Creative Questions
- Unlimited Question Paper
- Multiple Patterns & Answer keys
2221
1999



