- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students
Public Model Question Paper 2019 - 2020
12th Standard
-
Reg.No. :
Chemistry
Time :
02:45:00 Hrs
Total Marks :
70
-
Which metal is used for extraction of Au and Ag and also for galvanisation of iron objects?
(a)Mg
(b)Zn
(c)Cr
(d)Co
-
The basic structural unit of silicates is _______.
(a)\(\left( SiO_{ 3 } \right) ^{ 2- }\)
(b)\(\left( SiO_{ 4 } \right) ^{ 2- }\)
(c)\(\left( Sio \right) ^{ - }\)
(d)\(\left( SiO_{ 4 } \right) ^{ 4- }\)
-
The hybridisation and shape of SF6 is respectively?
(a)sp3d2, square planar
(b)sp3d2, octahedral
(c)sp3d see-saw
(d)sp3d, trigonal bipyramidal
-
Which of the following oxidation states is most common among the lanthanoids?
(a)+4
(b)+2
(c)+5
(d)+3
-
A complex has a molecular formula MSO4Cl.6H2O. The aqueous solution of it gives white precipitate with Barium chloride solution and no precipitate is obtained when it is treated with silver nitrate solution. If the secondary valence of the metal is six, which one of the following correctly represents the complex?
(a)[M(H2O)4Cl]SO4.2H2O
(b)[M(H2O)6]SO4
(c)[M(H2O)5Cl]SO4.H2O
(d)[M(H2O)3Cl]SO4.3H2O
-
In a solid, the cation is missing from the lattice and is found in an interstitial position. The defect is ________.
(a)Metal excess
(b)p type
(c)Schottky
(d)Frenkel
-
For a first order reaction A ⟶ B the rate constant is x min−1. If the initial concentration of A is 0.01M, the concentration of A after one hour is given by the expression.
(a)001. e−x
(b)1 x 10-2(1-e-60x)
(c)(1 x 10-2)e-60x
(d)none of these
-
If the solubility product of lead iodide is 3.2 × 10-8, its solubility will be _______.
(a)2 × 10-3M
(b)4 × 10-4M
(c)1.6 × 10-5M
(d)1.8 × 10-5M
-
The solution whose pH is maintained constant even upon the addition of small amounts of acid or base is called ________.
(a)acidic solution
(b)basic solution
(c)buffer solution
(d)true solution
-
The process in which chemical change occurs on passing electricity is termed as ______.
(a)neutralisation
(b)hydrolysis
(c)electrolysis
(d)ionisation
-
Fog is colloidal solution of _______.
(a)solid in gas
(b)gas in gas
(c)liquid in gas
(d)gas in liquid
-
Which among the following reagent is not used to differentiate ethanol and phenol?
(a)neutral FeCl3
(b)C6H5N2CI
(c)NaOH
(d)anhy ZnCl2
-
Reaction of acetone with one of the following reagents involves nucleophilic addition followed by elimination of water. The reagent is _______.
(a)Grignard reagent
(b)Sn / HCl
(c)hydrazine in presence of slightly acidic solution
(d)hydrocyanic acid
-
Which among the following exists as zwitter ion?
(a)sulphanilic acid
(b)salicylic acid
(c)acetanilide
(d)all the above
-
Pick out the odd one.
(a)Wax
(b)Starch
(c)Glucose
(d)Fructose
-
Write the two similarities between calcination and roasting.
-
What is catenation ? describe briefly the catenation property of carbon.
-
Discuss the oxidising power of fluorine.
-
Give the geometry and magnetic character of [NiCI4]2-
-
What are point defects?
-
Why is instantaneous rate preferred over average rate?
-
The concentration of hydroxide ion in a water sample is found to be 2.5 × 10-6M. Identify the nature of the solution.
-
Define conductance. Give its unit
-
Can we use nucelophiles such as NH3,CH3O - for the Nucleophilic substitution of alcohols
-
Explain alkali leaching in the extraction of aluminum.
-
Complete the following reactions?
i) Cr2 + 2e- ⟶
ii) Mn2+ + 2e- ⟶
iii) Fe2+ + 2e- ⟶
iv) CO2+ + 2e- ⟶ -
Write the reactions taking place in anode and cathode of a mercury button cell. Give the over all redox reaction of the cell with the emf generation
-
Describe some feature of catalysis by Zeolites.
-
Give chemical tests to distinguish between propan-2-ol and 2-methyl-propan-2-ol.
-
Identify A, B, C and D
\(\text { ethanoic acid } \stackrel{\mathrm{SOCl}_{2}}{\longrightarrow} \mathrm{A} \stackrel{\mathrm{Pd} / \mathrm{BaSO}_{4}}{\longrightarrow} \mathrm{B} \stackrel{\mathrm{NaOH}}{\longrightarrow} \mathrm{C} \stackrel {\longrightarrow}{\triangle} \mathrm{D}\) -
How can the following conversion be effected?
i) Nitrobenzene to anisole
ii) Chloro benzene to phenyl hydrazine
iii) Aniline to benzoic acid
iv) Benzene diazonium chloride to ehtyl benzene. -
Amino acids are amphoteric in nature. Explain.
-
Write a short note on Antioxidants.
-
-
Justify the following statement.
"Elements of the first transition series possess many properties different from those of heavier transition elements". -
Compound (A) of molecular formula C7H8 when treated with air in presence of V2O5 at 773 K gives a compound (B) of molecular formula C7H6O, which has the smell of bitter almonds. Alkaline KMnO4 oxidised compound (B) to (C) of molecular formula C7H6Or Compound (B) on treatment with N2H4 and KOH gives back compound (A). Identify (A), (B) & (C) and explain the reactions.
-
-
-
What are the general characteristics of solids?
-
The half life of the homogeneous gaseous reaction SO2Cl2 → SO2 + Cl2 which obeys first order kinetics is 8.0 minutes. How long will it take for the concentration of SO2Cl2 to be reduced to 1% of the initial value?
-
-
-
Write a note on zeolites.
-
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers.
(i) K[Cr(H2O)2 (C2O4)2]
(ii) [Co(en)3]CI3
(iii) [Co(NH3)5(NO2)](NO3)2
(iv) [Pt (NH3)(H2O)CI2]
-
-
-
Account for the following:
(i) Reducing character decreases from SO2 to TeO2.
(ii) Xenon forms compounds with fluorine and oxygen only. -
Explain the electrical property of colloids with a neat diagram. (or) Write a note on Helmholta electrical double layer.
-
-
-
Write the expression for the solubility product of Hg2Cl2 .
-
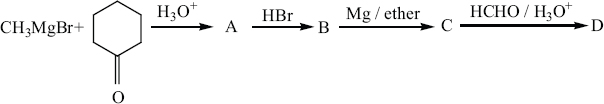
Identify A,B,C,D and write the complete equation
-
Part I
Answer all the questions.
Choose the most suitable answer
from the given four alternatives and write the option code with the
corresponding answer.
15 x 1 = 15
Part II
Answer any 6 questions. Question no. 24 is compulsory.
6 x 2 = 12
Part III
Answer any 6 questions. Question no. 33 is compulsory.
6 x 3 = 18
Part IV
Answer all the questions.
5 x 5 = 25






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Chemistry - Public Model Question Paper 2019 - 2020
Write your Comment