- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium - Important 5 Mark Question Paper and Answer Key 2022 - 2023 Study Materials Dec-31 , 2022
QB365 provides a detailed and simple solution for every Possible Questions in Class 12 Chemistry Subject - Important 5 Mark English Medium. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
12th Standard Chemistry Important 5 Mark Questions
12th Standard
-
Reg.No. :
Chemistry
Time :
01:30:00 Hrs
Total Marks :
150
-
Using the Ellingham diagram,
(A) Predict the conditions under which
(i) Aluminium might be expected to reduce magnesia.
(ii) Magnesium could reduce alumina.
(B) it is possible to reduce Fe2O3 by coke at a temperature around 1200K -
Give the limitations of Ellingham diagram.
-
Give the balanced equation for the reaction between chlorine with cold NaOH and hot NaOH.
-
Write the postulates of Werner’s theory.
-
On the basis of VB theory explain the nature of bonding in [Co(C2O4)3]3-
-
Differentiate crystalline solids and amorphous solids.
-
Calculate the percentage efficiency of packing in case of body centered cubic crystal.
-
What is an elementary reaction? Give the differences between order and molecularity of a reaction.
-
Discuss the Lowry – Bronsted concept of acids and bases.
-
State Kohlrausch Law. How is it useful to determine the molar conductivity of weak electrolyte at infinite dilution.
-
What is the difference between homogenous and hetrogenous catalysis?
-
Describe adsorption theory of catalysis
-
Predict the product A,B,X and Y in the following sequence of reaction
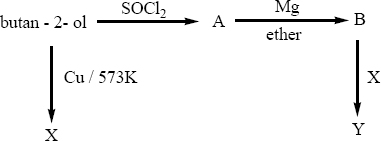
-
3,3 – dimethylbutan -2-ol on treatment with conc. H 2SO4 to give tetramethyl ethylene as a major product. Suggest a suitable mechanism.
-
Identify A, B, C and D
\(\text { ethanoic acid } \stackrel{\mathrm{SOCl}_{2}}{\longrightarrow} \mathrm{A} \stackrel{\mathrm{Pd} / \mathrm{BaSO}_{4}}{\longrightarrow} \mathrm{B} \stackrel{\mathrm{NaOH}}{\longrightarrow} \mathrm{C} \stackrel {\longrightarrow}{\triangle} \mathrm{D}\) -
An alkene (A) on ozonolysis gives propanone and aldehyde (B). When (B) is oxidised (C) is obtained. (C) is treated with Br2/P gives (D) which on hydrolysis gives (E). When propanone is treated with HCN followed by hydrolysis gives (E). Identify A, B, C, D and E.
-
Predict A,B,C and D for the following reaction
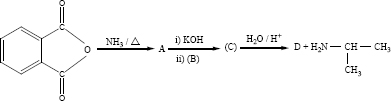
-
Identify A to E in the following sequence of reactions
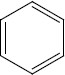
\(\overset { {CH_3} {CL} }{ \underset { {AlCl}_3 }{ \longrightarrow } }\) A \(\overset { {HNO_3}/ {H_2So_4} }{ \underset { {} }{ \longrightarrow } }\) B \(\overset { {Sn} /{HCL} }{ \underset {{} }{ \longrightarrow } }\) (C) \(\overset { {NaNo_2}/ {HCL} }{ \underset { {O^o}C }{ \longrightarrow } }\) D \(\overset { {CuCN} }{ \underset { {}}{ \longrightarrow } }\) E -
What are bio degradable polymers? Give examples.
-
How is terylene prepared?
-
Explain the preparation, properties, structure and uses of Diborane.
-
Complete the following reactions
(i) KCIO3\(\longrightarrow \)?
(ii) ZnS + O2\(\longrightarrow \) ?
(iii) Al2O3 + NaOH + H2O\(\longrightarrow \) ?
(iv) NaOH + SO2\(\longrightarrow \) ?
(v) KCI+ H2SO4 \(\longrightarrow \) ? -
Complete the following equations:
a. 4NaCI + MnO2 + 4H2SO4 \(\longrightarrow \)?
b. 6XeF4 + 12H2O\(\longrightarrow \) ? -
(i) \(\mathrm{PCl}_{5}+\mathrm{Ag} \longrightarrow \text { ? } \)
(ii) \(\mathrm{AgCl}_{(s)}+\mathrm{NH}_{3(\mathrm{aq})} \longrightarrow ? \)
(iii) \(\mathrm{PCl}_{3} \stackrel{\text { Moist air }}{\longrightarrow} \text { ? } \)
(iv) \(\mathrm{PCl}_{5} \stackrel{\text { Moist air }}{\longrightarrow} ? \)
(v) \(\mathrm{NaH}_{2} \mathrm{PO}_{2}+\mathrm{HCl} \longrightarrow ?\) -
Why is there a variation of atomic and ionic size as we move from Sc to Zn?
-
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers.
(i) K[Cr(H2O)2 (C2O4)2]
(ii) [Co(en)3]CI3
(iii) [Co(NH3)5(NO2)](NO3)2
(iv) [Pt (NH3)(H2O)CI2] -
Write note on impurity defect?
-
If 30% of a first order reaction is completed in 12 mins, what percentage will be completed in 65.33 mins?
-
Calculate the pH of solution with HO+ concentrations in mol dm-3.
(i) 10-4
(ii) 10-7
(iii) 6.8 x 10-3
(iv) 3.2 x 10-5
(v) 0.035
(vi) 0.25
(vii) 5.4 x 10-9
(viii) 7.1 x 10-7 -
The standard reduction potential for the reaction Sn4+ + 2e- ⟶ Sn2+ is + 0.15v. Calcuate the free energy change of the reaction.
Answer all the following questions.
30 x 5 = 150
*****************************************
Answers






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium - Important 5 Mark Question Paper and Answer Key 2022 - 2023
Write your Comment