- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium Reduced Syllabus Important Questions - 2021 Part - 1 Question Bank Software Jan-27 , 2021
12th Standard Chemistry English Medium Reduced Syllabus Important Questions - 2021 Part - 1
12th Standard Chemistry English Medium Reduced Syllabus Important Questions - 2021 Part - 1
12th Standard
-
Reg.No. :
Chemistry
Time :
02:45:00 Hrs
Total Marks :
165
-
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
(a)Fe
(b)Cu
(c)Mg
(d)Zn
-
Name the process by which elements such as germanium, silicon and gallium are refined.
(a)Vapour phase method
(b)Electrolytic refining
(c)Zone refining
(d)Van-Arkel method
-
The element that does not show catenation among the following p-block elements is ________.
(a)Carbon
(b)silicon
(c)Lead
(d)germanium
-
The repeating unit in silicone is_______.
(a)SiO2
(b)(c) (d)
(d)
-
The geometry at which carbon atom in diamond are bonded to each other is _______.
(a)Tetrahedral
(b)hexagonal
(c)Octahedral
(d)none of these
-
Column-I Column-II A Borazole 1 B(OH)3 B Boric acid 2 B3N3H6 C Quartz 3 Na2[B4O5(OH)4]8H2O D Borax 4 SiO2 (a)A B C D 2 1 4 3 (b)A B C D 1 2 4 3 (c)A B C D 1 2 4 3 (d)None of these
-
Thermodynamically the most stable form of carbon is________.
(a)Diamond
(b)graphite
(c)Fullerene
(d)none of these
-
Which one is correct statement for zeolite?
(a)Zeolites are aluminosilicates having three dimensional framework
(b)Hydrate zeolites are used as ion exchangers in hardening of soft water
(c)Zeolites are alumino silicates
(d)all the above
-
In graphite electrons are _______.
(a)localised on each C-atom
(b)localised on every third C-atom
(c)delocalised within the layer
(d)present in anti-bonding orbital
-
Silicones are ______.
(a)ortho silicates
(b)water repellent thermal insulators
(c)both (a) and (b)
(d)None of these
-
An element belongs to group 15 and 3rd period of the periodic table, its electronic configuration would be_______.
(a)1s2 2s2 2p4
(b)1s2 2s2 2p3
(c)1s2 2s2 2p6 3s2 3p2
(d)1s2 2s2 2p6 3s2 3p3
-
The correct order of the thermal stability of hydrogen halide is_______.
(a)HI > HBr > HCl > HF
(b)HF > HCl > HBr > HI
(c)HCl > HF > HBr > HI
(d)HI > HCl > HF > HBr
-
Most easily liquefiable gas is _______.
(a)Ar
(b)Ne
(c)He
(d)Kr
-
The catalytic activity of transition metals is due to ________.
(a)the formation of a variety of oxidation states
(b)the formation of intermediate products
(c)the capability of forming interstitial compounds.
(d)all the above
-
Which type of defect is found in transition metals that have variable valency?
(a)Frenkel defect
(b)Schottky defect
(c)Line defect
(d)Metal deficiency defect
-
What are the various steps involved in the extraction of pure metals from their ores?
-
What is the role of graphite rods in the electro metallurgy of aluminium?
-
Name and discuss the principle involved in obtaining silicon of high purity.
-
What are alums?
-
Starting from SiCl4, prepare the following in steps not exceeding the number given in parentheses.
(i) Silicon
(ii) Linear silicon containing methyl groups only
(iii) Na2SiO3 -
Chalcogens belongs to p-block. Give reason.
-
What are transition metals? Give four examples.
-
Name an element with which silicon can be doped to give an n-type semi conductor.
-
For a uni - univalent electrolyte write the Debye - Huckel Onsager equation
-
What is Helmholtz double layer?
-
Define Deemulsification.
-
What are lyophobic colloids? Give examples.
-
Explain the formation of delta.
-
Complete the following reaction giving names of products.
-
Predict the formula of the products in the
(i) CH3COCH3 + HCN ➝?
(ii) C6H5COCH + NH2OH➝? -
Using the Ellingham diagram,
(A) Predict the conditions under which
(i) Aluminium might be expected to reduce magnesia.
(ii) Magnesium could reduce alumina.
(B) it is possible to reduce Fe2O3 by coke at a temperature around 1200K -
AlCl3 behaves like a lewis acid. Substantiate this statement.
-
Write a note on metallic nature of p-block elements.
-
Account for the following:
(i) CO is used in the extraction of metals.
(ii) CO is poisonous
(iii) CO2 is used in refrigeration -
Give the uses of sulphuric acid.
-
Write the reason for the anomalous behaviour of Nitrogen.
-
Explain the variation in E0M3+/M2+ 3d series.
-
Compare lanthanoids and actinoids.
-
Classify the following ligands based on the number of donor atoms.
a) NH3
b) en
c) ox2-
d) pyridine -
Explain Schottky defect.
-
Classify the following solids in different categories based on the nature of intermolecular force operating in them: Potassium sulphate, tin, benzene, urea, ammonia, water, zinc sulphide, graphite, rubidium, argon, silicon carbide.
-
Write Arrhenius equation and explains the terms involved.
-
Hydrolysis of methyl acetate in aqueous solution has been studied by titrating the liberated acetic acid against sodium hydroxide. The concentration of an ester at different temperatures is given below.
t(min) 0 20 40 60 ∝ v (ml) 20.2 25.6 29.5 32.8 50.4 Show that the reaction is the first order reactions.
-
Two metals M1 and M2 have reduction potential values of -xV and +yV respectively. Which will liberate H2 and H2SO4.
-
How are colloidal solution of ink and graphite prepared?
-
Describe the role of the following in the process mentioned.
(i) Silica in the extraction of copper.
(ii) Cryolite in the extraction of aluminium.
(iii) Iodine in the refining of Zirconium.
(iv) Sodium cyanide in froth floatation. -
Write a short note on electrochemical principles of metallurgy.
-
How can you separate alumina from silica in a bauxite ore.
-
How are silicates classified? Give an example for each type of silicate.
-
What type of hybridisation occur in
a) BrF5
b) BrF3 -
How does sulphuric acid react with metals at various conditions.
-
Transition metals show high melting points. Why?
-
What is the coordination entity formed when excess of liquid ammonia is added to an aqueous solution of copper sulphate?
-
Atoms X and Y form bcc crystalline structure. Atom X is present at the corners of the cube and Y is at the centre of the cube. What is the formula of the compound?
-
From the following data, show that the decomposition of hydrogen peroxide is a reaction of the first order:
t(min) 0 10 20 V(ml) 46.1 29.8 19.3 Where t is the time in minutes and V is the volume of standard KMnO4 solution required for titrating the same volume of the reaction mixture.
-
Write an account of the Arrhenius equation for rates of chemical reactions.
-
Complete the following reactions
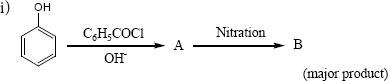
ii) \(C_6H_5-CH_{2}CH(OH)CH(CH_3)_2 \overset{ConH_2SO_4}\longrightarrow\) -
An organic compound (A) (C6H6O) gives maximum of two isomers (B) and (C) When an alkaline solution of (A) is refluxed with chloroform. (B) on oxidation gives acid (D). The acid (D) is also obtained by I treating sodium salt of (A) with CO2 under pressure followed by hydrolysis. Identify the compounds (A), (B), (C) and (D) and explain with proper chemical reactions.
-
Compound (A) with molecular formula C6H6O gives violet colour with neutral FeCI3 (A) reacts with CHCl3 and NaOH gives two isomers (B) and (C) with molecular formula C7H6O2 Compound (A) reacts with ammonia at 473 K in the presence of ZnCl2 gives compound (D) with molecular formula C6H7N. Compound (D) undergoes carbylamine test. Identify (A), (B), (C) and (D) and explain the reactions.
-
An organic compound (A) of molecular formula C6H6O gives violet colour with neutral FeCI3. (A) gives maximum of two isomers (B) and (C) when an alkaline solution of (A) is refluxed with CCI4 (A) also reacts with C6H5N2CI to give the compound (D) which is red orange dye. Identify (A), (B), (C) and (D). Explain with suitable chemical reactions.
Multiple Choice Questions
15 x 1 = 15
2 Marks
15 x 2 = 30
3 Marks
15 x 3 = 45
5 Marks
15 x 5 = 75






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium Reduced Syllabus Important Questions - 2021 Part - 1
Write your Comment