- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry Public Model Question Paper I 2019 - 2020 Question Bank Software Feb-21 , 2020
12th Standard Chemistry Public Model Question Paper I 2019 - 2020
Public Model Question Paper Part-II 2019 - 2020
12th Standard
-
Reg.No. :
Chemistry
Time :
02:45:00 Hrs
Total Marks :
70
-
Name the process by which elements such as germanium, silicon and gallium are refined.
(a)Vapour phase method
(b)Electrolytic refining
(c)Zone refining
(d)Van-Arkel method
-
An aqueous solution of borax is________.
(a)neutral
(b)acidic
(c)basic
(d)amphoteric
-
Pick the wrong one among the following
(a)F2 - Yellow
(b)Br2 - Red
(c)Cl2 - Colourless
(d)I2- Violet
-
Which one of the following ions has the same number of unpaired electrons as present in V3+?
(a)Ti3+
(b)Fe3+
(c)Ni2+
(d)Cr3+
-
A complex in which the oxidation number of the metal is zero is_______.
(a)K4[Fe(CN)6]
(b)[Fe(CN)3(NH3)3]
(c)[Fe(CO)5]
(d)both (b) and (c)
-
The number of close neighbours in a body centred cubic lattice of identifical spheres is _______.
(a)6
(b)4
(c)12
(d)8
-
The half life period of a radioactive element is 140 days. After 560 days, 1 g of element will be reduced to
(a)\(\left( \frac { 1 }{ 2 } \right) g\)
(b)\(\left( \frac { 1 }{ 4 } \right) g\)
(c)\(\left( \frac { 1 }{ 8 } \right) g\)
(d)\(\left( \frac { 1 }{ 16 } \right) g\)
-
Which of these is not likely to act as Lewis base?
(a)BF3
(b)PF3
(c)CO
(d)F–
-
The hydrogen ion concentration of a buffer solution consisting of a weak acid and its salt is given by _______.
(a)\([{ H }^{ + }]={ K }_{ a }\frac { [Acid] }{ [Salt] } \)
(b)\([{ H }^{ + }]={ K }_{ a }[Salt]\)
(c)\([{ H }^{ + }]={ K }_{ a }[Salt]\)
(d)\([{ H }^{ + }]={ K }_{ a }\frac { [Acid] }{ [Salt] } \)
-
The overall reaction that takes place in an electrochemical cell is ______.
(a)oxidation
(b)reduction
(c)decomposition
(d)redox reaction
-
Which one of the following is correctly matched?
(a)Emulsion - Smoke
(b)Gel - butter
(c)foam - Mist
(d)whipped cream - sol
-
The IUPAC name for isobutyl alcohol is _______.
(a)2-methyl-l-propanol
(b)2-methyl-l-butanol
(c)2,2-dimethyl-2-propanol
(d)1,1-dimethyl- 2-butanol
-
Which of the following represents the correct order of acidity in the given compounds
(a)FCH2COOH > CH3COOH > BrCH2COOH > ClCH2COOH
(b)FCH2COOH > ClCH2COOH > BrCH2COOH > CH3COOH
(c)CH3COOH > ClCH2COOH > FCH2COOH > Br-CH2COOH
(d)Cl CH2COOH > CH3COOH > BrCH2COOH > ICH2COOH
-
Methylisocyanide on reduction using LiAlH4 gives:
(a)Methyl amine
(b)Ethyl amine
(c)Dimethyl amine
(d)Trimethyl amine
-
At pH 12, glycine exists as
(a)H3N+ - CH2 - COOH
(b)H2N - CH2 - COO-
(c)H3N+ - CH2- COO-
(d)H2N - CH2 - COOH
-
What is meant by concentration?
-
Give the uses of Borax
-
Account for the following:
(i) O-O bond lengths in ozone molecule are identical.
(ii) Most of the reactions in fluorine are exothermic. -
Write the IUPAC name of [Ag(NH3)2]Cl.
-
Give any three characteristics of ionic crystals.
-
Mention the factors that affected the rate of a chemical reaction.
-
Identify the conjugate acid base pair for the following reaction in aqueous solution
i) HS- (aq) + HF \(\rightleftharpoons \) F-(aq) + H2S(aq)
ii) HPO2-4 + SO32- \(\rightleftharpoons \) PO43- + HSO3-
iii) NH4+ + CO32- \(\rightleftharpoons \) NH3 + HCO3- -
Explain the function of a salt bridge in an electrochemical cell.
-
Arrange the following in the increasing order of their boiling point and give a reason for your ordering
(i) Butan – 2- ol, Butan -1-ol, 2 –methylpropan -2-ol
(ii) Propan -1-ol, propan -1,2,3-triol, propan -1,3 – diol, propan -2-ol -
Write the chemical composition of the following alloys and give anyone of its application.
(i) Bronze
(ii) Brass
(iii) Stainless steel -
Complete the following reactions?
i) Cr2 + 2e- ⟶
ii) Mn2+ + 2e- ⟶
iii) Fe2+ + 2e- ⟶
iv) CO2+ + 2e- ⟶ -
Corrosion of aluminum takes place at a much slower rate than iron. Give reason
-
Peptising agent is added to convert precipitate into colloidal solution. Explain with an example.
-
Account for the following:
(i) Phenol has a smaller dipole moment than methanol,
(ii) Phenols do not give protonation reaction readily. -
An alkene (A) on ozonolysis gives propanone and aldehyde (B). When (B) is oxidised (C) is obtained. (C) is treated with Br2/P gives (D) which on hydrolysis gives (E). When propanone is treated with HCN followed by hydrolysis gives (E). Identify A, B, C, D and E.
-
How will convert nitrobenzene to benzoic acid?
-
Write a reaction that indicates the presence of an aldehyde group in glucose.
-
Give the structure of melamine formaldehyde resin.
-
Write a note on zeolites.
-
How is chlorine manufactured by the electrolysis of brine.
-
Justify the following statement.
"Elements of the first transition series possess many properties different from those of heavier transition elements". -
What is meant by stability of a co-ordination compound in solution? State the factors which govern stability of complexes.
-
How are crystals classified?
-
A zero order reaction is 20% complete in 20 minutes. Calculate the value of the rate constant. In what time will the reaction be 80% complete?
-
Write the expression for the solubility product of Hg2Cl2 .
-
Give the special characteristics of enzyme catalysed reactions.
-
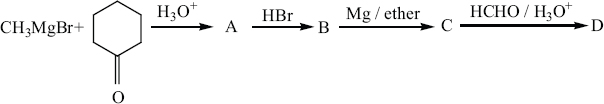
Identify A,B,C,D and write the complete equation -
An organic compound (A) of molecular formula C7H8 on oxidation with air and in presence of V2O5 to form (B) of molecular formula C7H6O. (B) on reduction with lithium aluminium hydride to form (C) of molecular formula C7H8O. Identify (A), (B) and (C) and explain the reactions.
Part I
Answer all the questions.
Choose the most suitable answer from the given four alternatives and write the option code with the corresponding answer.
15 x 1 = 15
Part II
Answer any 6 questions. Question no. 24 is compulsory.
9 x 2 = 18
Part III
Answer any 6 questions. Question no. 33 is compulsory.
9 x 3 = 27
Part IV
Answer all the questions.
10 x 5 = 50






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry Public Model Question Paper I 2019 - 2020
Write your Comment