- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry Public Model Question Paper IV 2020 Question Bank Software Feb-21 , 2020
12th Standard Chemistry Public Model Question Paper IV 2020
Public Model Question Paper 2020 - 2021
12th Standard
-
Reg.No. :
Chemistry
Time :
02:45:00 Hrs
Total Marks :
70
-
In acid leaching process the insoluble sulphide is converted into soluble sulphate and elemental_______.
(a)carbon
(b)lead
(c)sulphur
(d)zinc
-
Oxidation state of carbon in its hydrides _______.
(a)+4
(b)-4
(c)+3
(d)+2
-
Repeated use of which one of the following fertilizers would increase the activity of the soil_______.
(a)Ammonium sulphate
(b)Superphosphate of lime
(c)Urea
(d)Potassium nitrate
-
The magnetic moment of Mn2+ ion is _______.
(a)5.92BM
(b)2.80BM
(c)8.95BM
(d)3.90BM
-
A magnetic moment of 1.73BM will be shown by one among the following.
(a)TiCl4
(b)[CoCl6]4-
(c)[Cu(NH3)4]2+
(d)[Ni(CN)4]2-
-
The number of close neighbours in a body centred cubic lattice of identifical spheres is _______.
(a)6
(b)4
(c)12
(d)8
-
The half life period of a radioactive element is 140 days. After 560 days, 1 g of element will be reduced to
(a)\(\left( \frac { 1 }{ 2 } \right) g\)
(b)\(\left( \frac { 1 }{ 4 } \right) g\)
(c)\(\left( \frac { 1 }{ 8 } \right) g\)
(d)\(\left( \frac { 1 }{ 16 } \right) g\)
-
Which of the following can act as Lowery – Bronsted acid well as base?
(a)HCl
(b)SO42−
(c)HPO42−
(d)Br-
-
The hydrogen ion concentration of a buffer solution consisting of a weak acid and its salt is given by _______.
(a)\([{ H }^{ + }]={ K }_{ a }\frac { [Acid] }{ [Salt] } \)
(b)\([{ H }^{ + }]={ K }_{ a }[Salt]\)
(c)\([{ H }^{ + }]={ K }_{ a }[Salt]\)
(d)\([{ H }^{ + }]={ K }_{ a }\frac { [Acid] }{ [Salt] } \)
-
Using the data given below find out the strongest reducing agent ______.
\({ E }_{ { Cr }_{ 2 }{ O }_{ 7 }^{ 2- } }^{ o }{ Cr }^{ 3+ }=1.33V{ ,E }_{ { Cl }_{ 2 }{ / }{ Cl }^{ - } }^{ o }=1.36V\)
\({ E }_{ { Mn }O_{ 4 }^{ - } }^{ 0 }/{ Mn }^{ 2+ }=1.51V,{ E }_{ { Cr }^{ 3+ }/Cr }^{ o }=-0.74V\)(a)Cr
(b)Cr3+
(c)Cl-
(d)Mn2+
-
The phenomenon observed when a beam of light is passed through a colloidal solution is_______.
(a)Cataphoresis
(b)Electrophoresis
(c)Coagulation
(d)Tyndall effect
-
Ethers are insoluble in water due to the ______.
(a)absence of co-ordinate bond
(b)presence of co-ordinate bond
(c)absence of H - bond
(d)presence of H - bond
-
An alkene “A” on reaction with O3 and Zn - H2O gives propanone and ethanol in equimolar ratio. Addition of HCl to alkene “A” gives “B” as the major product. The structure of product “B” is ______.
(a)\(Cl-{ CH }_{ 2 }-CH_{ 2 }-\overset { \overset { CH_{ 3 } }{ | } }{ \underset { \underset { CH_{ 3 } }{ | } }{ CH } } \)
(b)\({ H }_{ 3 }C-{ CH }_{ 2 }-\overset { \overset { CH_{ 2 }Cl }{ | } }{ CH } -{ CH }_{ 3 }\)
(c)\(\\ { H }_{ 3 }C-{ CH }_{ 2 }-\overset { \overset { CH_{ 3 } }{ | } }{ \underset { \underset { CL{ } }{ | } }{ C } } -{ CH }_{ 3 }\)
(d)\({ H }_{ 3 }C-{ CH }-\overset { \overset { CH_{ 3 } }{ | } }{ \underset { \underset { Cl }{ | } }{ C } } \)
-
Which one of the following is a secondary amine?
(a)aniline
(b)diphenyl amine
(c)see.butylamine
(d)tert.butylamine
-
Name the various refining process.
-
Give the uses of Borax
-
Give two uses of nitric acid.
-
Calculate the magnetic moment of [Fe(H2O)6]2+, if atomic number of Fe is 26.
-
Arrange the following as indicated below:
(i) F2, Cl2, Br2, I2 - increasing bond dissociation enthalpy.
(ii) HF, HCI, HBr, HI - increasing acidic strength. -
How will you determine the rate constant for the Decomposition of nitrogen pentoxide in CCl4.
-
Discuss the Lowry – Bronsted concept of acids and bases.
-
Give the empirical relationship between molar conductance and concentration of the electrolyte.
-
Suggest a suitable reagent to prepare secondary alcohol with identical group using Grignard reagent.
-
Write the chemical composition of the following alloys and give anyone of its application.
(i) Bronze
(ii) Brass
(iii) Stainless steel -
What is chromyl chloride test? Give equations.
-
How to predict the feasibility of a cell reaction?
-
Examine the given defective crystal:
X+ Y- X+ Y- X+ Y- O X+ X+ X+ O X+ Y- X+ Y- X+ Y- Answer the following questions
(i) Is the above defect stoichiometric or non-stoichiometric?
(ii) What are such defects called? Give an example of the compound which shows this type of defect. -
Account for the following:
(a) Lower members of alcohols are soluble in water but higher members are not.
(b) Alcohols cannot be used as solvent for Grignard reagent. -
An alkene (A) on ozonolysis gives propanone and aldehyde (B). When (B) is oxidised (C) is obtained. (C) is treated with Br2/P gives (D) which on hydrolysis gives (E). When propanone is treated with HCN followed by hydrolysis gives (E). Identify A, B, C, D and E.
-
Write a note on structure of amine.
-
Give the structure of α - D - glucose and β- D - glucose
-
Give examples for
(i) addition polymer,
(ii) Synthetic rubbers,
(iii) Condensation polymers,
(iv) formaldehyde resins. -
-
Complete the following reactions
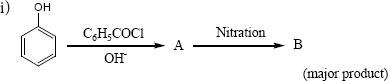
ii) \(C_6H_5-CH_{2}CH(OH)CH(CH_3)_2 \overset{ConH_2SO_4}\longrightarrow\) -
An aromatic aldehyde (A) of molecular formula C7H6O which has the smell of bitter almonds on treatment with (CH3CO)2O and CH3COONa to give compound (B) which is an aromatic unsaturated add. (A) also reacts with (A) in the presence of ale. KCN to give dimer (C). Identify (A), (B) and (C). Explain the reactions.
-
-
-
A hydride of 2nd period alkali metal (A) on reaction with compound of Boron (B) to give a reducing agent (C). Identify A, B and C.
-
An amorphous solid (A) burns in air to form a gas (B) which turns lime water milky. The gas is also produced as a byproduct during roasting of sulphide ore. This gas decolourises acidified aqueous KMnO4 solution and reduces Fe3+ to Fe2+. Identify the solid 'A' and the gas 'B' and write the reactions involved.
-
-
-
Write the expression for the solubility product of Hg2Cl2 .
-
Write a note on Freundlich adsorption isotherm.
-
-
-
In a pseudo first order hydrolysis of ester in water, the following results were obtained.
1 0 30 60 90 [Ester]mol L-1 0.55 0.31 0.17 0.085 (i) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(i) Calculate the pseudo first order rate constant for the hydrolysis of ester. -
How do nature of the reactant influence rate of reaction.
-
-
-
Write a short note on the oxidation states of 3d series elements.
-
What is meant by stability of a co-ordination compound in solution? State the factors which govern stability of complexes.
-
Part I
Answer all the questions.
Choose the most suitable answer from the given four alternatives and write the option code with the corresponding answer.
15 x 1 = 15
Part II
Answer any 6 questions. Question no. 24 is compulsory.
6 x 2 = 12
Part III
Answer any 6 questions. Question no. 33 is compulsory.
6 x 3 = 18
Part IV
Answer all the questions.
5 x 5 = 25






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry Public Model Question Paper IV 2020
Write your Comment