- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium Reduced Syllabus Model Question paper - 2021 Part - 1 Question Bank Software Jan-27 , 2021
12th Standard Chemistry English Medium Reduced Syllabus Model Question paper - 2021 Part - 1
12th Standard Chemistry English Medium Reduced Syllabus Model Question paper - 2021 Part - 1
12th Standard
-
Reg.No. :
Chemistry
Time :
02:45:00 Hrs
Total Marks :
70
-
Zinc is obtained from ZnO by________.
(a)Carbon reduction
(b)Reduction using silver
(c)Electrochemical process
(d)Acid leaching
-
Which of the following is not true with respect to Ellingham diagram?
(a)Free energy changes follow a straight line. Deviation occurs when there is a phase change.
(b)The graph for the formation of CO2 is a straight line almost parallel to free energy axis.
(c)Negative slope of CO shows that it becomes more stable with increase in temperature.
(d)Positive slope of metal oxides shows that their stabilities decrease with increase in temperature.
-
Oxidation state of carbon in its hydrides _______.
(a)+4
(b)-4
(c)+3
(d)+2
-
Which of the following is strongest acid among all?
(a)HI
(b)HF
(c)HBr
(d)HCl
-
Which one of the following statements related to lanthanons is incorrect?
(a)Europium shows +2 oxidation state
(b)The basicity decreases as the ionic radius decreases from Pr to Lu.
(c)All the lanthanons are much more reactive than aluminium
(d)Ce4+ solutions are widely used as oxidising agents in volumetric analysis.
-
Which one of the following is not correct?
(a)La(OH)3 is less basic than Lu(OH)3
(b)In lanthanoid series ionic radius of Ln3+ ions decreases
(c)La is actually an element of transition metal series rather than lanthanide series
(d)Atomic radii of Zr and Hf are same because of lanthanide contract
-
A complex in which the oxidation number of the metal is zero is_______.
(a)K4[Fe(CN)6]
(b)[Fe(CN)3(NH3)3]
(c)[Fe(CO)5]
(d)both (b) and (c)
-
An ionic compound Ax By crystallizes in fcc type crystal structure with B ions at the centre of each face and A ion occupying corners of the cube the correct formula of Ax, By is ________.
(a)AB
(b)AB3
(c)A3B
(d)A8B6
-
The number of carbon atoms per unit cell of diamond is _______.
(a)8
(b)6
(c)1
(d)4
-
The fraction of total volume occupied by the atoms in a simple cubic is ________.
(a)\(\left( \frac { \pi }{ 4\sqrt { 2 } } \right) \)
(b)\(\left( \frac { \pi }{ 6 } \right) \)
(c)\(\left( \frac { \pi }{ 4 } \right) \)
(d)\(\left( \frac { \pi }{ 3\sqrt { 2 } } \right) \)
-
Among the following graphs showing variation of rate constant with temperature (T) for a reaction, the one that exhibits Arrhenius behavior over the entire temperature range is _______.
(a) (b)
(b) (c)
(c) (d)
(d)both (b) and (c)
-
Predict the rate law of the following reaction based on the data given below
2A+B⟶C+3DReaction number [A] (min) [B] (min) Initial rate (M s-1) 1 0.1 0.1 x 2 0.2 0.1 2x 3 0.1 0.2 4x 4 0.2 0.2 8x (a)rate = k[A]2 [B]
(b)rate = k[A] [B]2
(c)rate = k[A] [B]
(d)rate = k[A]1/2 [B]3/2
-
This reaction follows first order kinetics. The rate constant at particular temperature is 2.303 x 10-2 hour-1. The initial concentration of cyclopropane is 0.25 M. What will be the concentration of cyclopropane after 1806 minutes? (log 2 = 0.3010)
(a)0.125 M
(b)0.215 M
(c)0.25 x 2.303 M
(d)0.05 M
-
Using Gibb’s free energy change, ∆Go=57.34 kJ mol-1, for the reaction, X2Y(s)⇌2X++Y2- (aq), calculate the solubility product of X2Y in water at 300 K_______. (R = 8.3 J K-1Mol-1)
(a)10-10
(b)10-12
(c)10-14
(d)can not be calculated from the given dat
-
The button cell used is watches function as follows
Zn (s) + Ag2O (s) + H2O (l) ⇌ 2Ag (s) + Zn2+ (aq) + 2OH-(aq) the half cell potentials are Ag2O (s) + H2O (l) + 2e- → 2Ag (s) + 2OH- (aq) Eo = 34V and Zn (s) → Zn2+ (aq) + 2e− E0 = 0.76V . The cell potential will be_______.(a)0.84V
(b)1.34V
(c)1.10V
(d)0.42V
-
Define Deemulsification.
-
Name the types of emulsions.
-
Why sodium metal cannot be used to dry alcohols but it can be used to dry ethers?
-
How will you distinguish between formaldehyde and acetaldehyde?
-
Give the structural formula of
i) 2-amino-2-methyl propane
ii) 2-(N, N- dimethyl) amino butane -
\({ C }_{ 6 }{ H }_{ 5 }{ NH }_{ 2 }\xrightarrow [ 273k,HCl ]{ { HNO }_{ 2 } } A\xrightarrow [ { Cu }_{ 2 }({ CN })_{ 2 } ]{ KCN } B \stackrel { { H }_{ 3 }O }\rightarrow C\)
Identify A, Band C. -
Use the following reagents in the correct order and bring about the conversion of benzene to aniline.
Reagents - HCI, NH3, heat, alk KMnO4, CHCl3 / AlCl3, Br2/KOH that can be used. -
Give the IUPAC name of the following compounds
i) \({ H }_{ 3 }C-\underset { \overset { | }{ CN } }{ CH } -{ CH }_{ 2 }-COOH\)
ii) \({ CH }_{ 3 }-\underset { \overset { | }{ { CH }_{ 3 } } }{ CH } -CN\)
iii) C6H5-NC -
Name the vitamin responsible for coagulation of blood.
-
Give examples for first order reaction.
-
Explain the relationship between free energy of the cell and its emf.
-
Write a note on Ultrafilteration?
-
Complete the following equations by writing the missing A, B, C, D etc.,
-
Write short notes on Popoff's rule.
-
How are the following compounds obtained from benzene diazonium chloride?
(i) phenol
(ii) ester
(iii) p-hydroxy azo benzene -
ExpIain the classification of proteins based on their structure.
-
Write a note on tertiary structure of proteins.
-
Give the structure of sucrose.
-
Explain the principle of electrolytic refining with an example.
-
How will you identify borate radical?
-
How are silicates classified? Give an example for each type of silicate.
-
The E0M2+/M value for copper is positive. Suggest a possible reason for this.
-
Why first ionization enthalpy of chromium is lower than that of zinc?
-
Aluminium crystallizes in a cubic close packed structure. Its metallic radius is 125pm. calculate the edge length of unit cell.
-
The time for half change in a first order decomposition of a substance A is 60 seconds. Calculate the rate constant. How much of A will be left after 180 seconds?
-
Write the expression for the solubility product of Hg2Cl2 .
-
9.2\(\times\)1012 litres of water is available in a lake. A power reactor using the electrolysis of water in the lake, produces electricity at the rate of 2\(\times\)106 Cs−1 at an appropriate voltage. How many years would it take to completely electrolyse the water in the lake. Assume that there is no loss of water except due to electrolysis.
-
Complete the following reactions
i) CH3- CH2 - OH \(\overset { { P}{ Br_3 }{ } }{ \underset { {} }{ \longrightarrow } }\) A \(\overset { { aq.NaOH}{ }{ } }{ \underset { {} }{ \longrightarrow } }\) B \(\overset { { Na}{ } }{ \underset { {} }{ \longrightarrow } }\) C
ii) C6H5- OH \(\overset { { Zn \ dust}{ } }{ \underset { {} }{ \longrightarrow } }\) A \(\overset { { CH_3}{Cl}{ } }{ \underset { {Anhydrs}{AlCl_3} }{ \longrightarrow } }\) B \(\overset { { acid}{K MnO_4}{ } }{ \underset { {} }{ \longrightarrow } }\) C
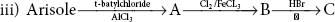
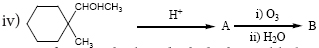
Part I
Answer all the questions.
Choose the most suitable answer from the given four alternatives and write the option code with the corresponding answer.
15 x 1 = 15
Part II
Answer any 6 questions. Question no. 24 is compulsory.
9 x 2 = 18
Part III
Answer any 6 questions. Question no. 33 is compulsory.
9 x 3 = 27
Part IV
Answer all the questions.
10 x 5 = 50






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium Reduced Syllabus Model Question paper - 2021 Part - 1
Write your Comment