- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium Reduced Syllabus Model Question paper - 2021 Part - 2 Question Bank Software Jan-27 , 2021
12th Standard Chemistry English Medium Reduced Syllabus Model Question paper - 2021 Part - 2
12th Standard Chemistry English Medium Reduced Syllabus Model Question paper - 2021 Part - 2
12th Standard
-
Reg.No. :
Chemistry
Time :
02:45:00 Hrs
Total Marks :
70
-
Which of the following statements, about the advantage of roasting of sulphide ore before reduction is not true?
(a)\(\Delta { G }_{ f }^{ 0 }\) of sulphide is greater than those for CS2 and H2S
(b)\(\Delta { G }_{ r }^{ 0 }\) is negative for roasting of sulphide ore to oxide
(c)Roasting of the sulphide to its oxide is thermodynamically feasible
(d)Carbon and hydrogen are suitable reducing agents for metal sulphides
-
Extraction of gold and silver involves leaching with cyanide ion. silver is later recovered by_______.
(a)Distillation
(b)Zone refining
(c)Displacement with zinc
(d)liquation
-
Which of the following plot gives Ellingham diagram
(a)\(\Delta S \ \text{Vs} \ T\)
(b)\(\Delta { G }^{ 0 }\ \text{Vs} \ T\)
(c)\(\Delta { G }^{ 0 }\ \text{Vs} \ \frac { 1 }{ T } \)
(d)\(\Delta { G }^{ 0 }\ \text{Vs} \ { T }^{ 2 }\)
-
The basic structural unit of silicates is _______.
(a)\(\left( SiO_{ 3 } \right) ^{ 2- }\)
(b)\(\left( SiO_{ 4 } \right) ^{ 2- }\)
(c)\(\left( Sio \right) ^{ - }\)
(d)\(\left( SiO_{ 4 } \right) ^{ 4- }\)
-
XeF6 on complete hydrolysis produces________.
(a)XeOF4
(b)XeO2F2
(c)XeO3
(d)XeO2
-
Which is the group that decreases the acid strength of phenol?
(a)-NO2
(b)-CN-
(c)-NH2
(d)Both (a) and (b)
-
The compound that acts as a solvent for Grignard reagent is ______.
(a)Ethyl alcohol
(b)Diethyl ether
(c)Acetone
(d)Benzene
-
\(\overset { { CH }_{ 2 }-OH }{ \underset { { CH }_{ 2 }-OH }{ | } } \overset { { PI }_{ 3 } }{ \longrightarrow } \) __________ethan-1,2-diol
(a)ethane
(b)ethene
(c)ethyl iodide
(d)ethyne
-
The ether used in perfumery is ________.
(a)diethyl ether
(b)dimethyl ether
(c)methyl phenyl ether
(d)diphenyl ether
-
The active component of dynamite is _______.
(a)keiselghur
(b)nitro glycerine
(c)nitro benzene
(d)trinitro toluene
-
Nylon -66 is an example of ______ polymer.
(a)addition
(b)condensation
(c)both (a) and (b)
(d)natural
-
Teflon is an _______ polymer.
(a)LDPE
(b)HDPE
(c)Condensation
(d)All the above
-
The quality of a soap is described in term of _______
(a)LDPE
(b)HDPE
(c)TFM (Total fatty matter)
(d)LFM (Lowfatty matter)
-
Pain killers are known a _______
(a)antipyretics
(b)analgesics
(c)antibiotics
(d)antiseptics
-
Iodoform is ________
(a)antibiotic
(b)antiseptic
(c)disinfectant
(d)both (a) and (c)
-
Write a short note on anomalous properties of the first element of p-block.
-
Why fluorine is more reactive than other halogens?
-
Define average rate and instantaneous rate.
-
Give the IUPAC names of
(i) CH3CH(OH)CH2OH
(ii) HO-CH2-CH2-OH
(iii) \({ { CH } }_{ 3 }-\underset { \overset { | }{ OH } }{ CH } -COOH\) -
Write the IUPAC names of
(a) C2H5OCH3
(b) C6H5OC2H5 -
Which of the following does not give iodoform reaction?
-
Write any three uses of formic acid.
-
Write a note on Claisen Condensation.
-
Identify A and B in the following sequence of reactions.\({ CH }_{ 3 }-Br\xrightarrow [ 675k\\ Red\ hot\ si\ tube ]{ { HNO }_{ 3 } } A\xrightarrow [ NaOH ]{ { Cl }_{ 2 } } B\)
-
Copper and silver lie low in the electrochemical series and yet they are found in the combined state as sulphides in nature. Comment.
-
Write the molecular formula and structural formula for the following molecules.
a) Nitric acid
b) Dinitrogen pentoxide
c) phosphoric acid (PTA)
d) phosphine -
Define ionic product of water. Give its value at room temperature.
-
The conductivity of a 0.01M solution of a 1 :1 weak electrolyte at 298K is 1.5\(\times\)10-4 S cm−1.
i) molar conductivity of the solution
ii) degree of dissociation and the dissociation constant of the weak electrolyte
Given that
\(\lambda^{0}_{cation}=248.2 \ S\) cm2 mol-1
\(\lambda^{0}_{anlon}=51.8 \ S\) cm2 mol-1 -
Arrange the following solutions in the decreasing order of specific conductance.
i) 0.01M KCl
ii) 0.005M KCl
iii) 0.1M KCl
iv) 0.25M KCl
v) 0.5M KCl -
What are enzymes? Write a brief note on the mechanism of enzyme catalysis.
-
Arrange the following
i. In increasing order of solubility in water, C6H5 NH2, (C2H5)2NH, C2H5NH2
ii. In increasing order of basic strength
a) aniline, p- toludine and p – nitroaniline
b) C6H5 NH2, C6H5 NHCH3, C6H5NH2, p-Cl-C6- H4-NH2
iii. In decreasing order of basic strength in gas phase
(C2H5)NH2, (C2H5)NH, (C2H5)5N and NH3
iv. In increasing order of boiling point
C6H5OH, (CH3)2NH, C2H5NH2
v. In decreasing order of the pKb values
C2H5NH2, C6H5NHCH3.(C2H5)2 NH and CH3NH2
vi. Increasing order of basic strength
C2H5NH2,C6H5N(CH3)2, (C2H5)2 NH and CH3NH2
vii. In decreasing order of basic strength
-
How will you prepare propan – 1- amine from
i) butane nitrile
ii) propanamide
ii) 1- nitropropane -
Explain the mechanism of cleansing action of soaps and detergents.
-
Why do zirconium and Hafnium exhibit similar properties?
-
Which metal in the 3d series exhibits +1 oxidation state most frequently and why?
-
Atoms X and Y form bcc crystalline structure. Atom X is present at the corners of the cube and Y is at the centre of the cube. What is the formula of the compound?
-
Predict the product A,B,X and Y in the following sequence of reaction
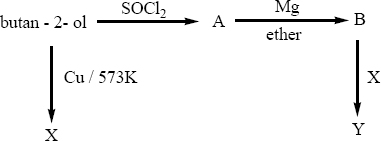
-
Ail organic compound. (A) C7H6O reduces Tollens reagent. Compound (A) reacts with acetic anhydride in the presence of anhydrous sodium acetate and gives an unsaturated acid (B). Compound (A) reacts with acetone in the presence of alkali and gives (C). What are (A)(B) and (C) Explain the reactions.
-
What happens when ethylamine is treated with
(i) CHCl3/NaOH
(ii) CS2
(iii) C6H5CHO. -
Write the differences between nitro methane and nitro benzene.
-
List the importance of proteins in biological processes.
-
What are narcotic and non – narcotic drugs. Give examples
-
Write a note on drug target interaction.
Part I
Answer all the questions.
Choose the most suitable answer from the given four alternatives and write the option code with the corresponding answer.
15 x 1 = 15
Part II
Answer any 6 questions. Question no. 24 is compulsory.
9 x 2 = 18
Part III
Answer any 6 questions. Question no. 33 is compulsory.
9 x 3 = 27
Part IV
Answer all the questions.
10 x 5 = 50






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium Reduced Syllabus Model Question paper - 2021 Part - 2
Write your Comment