- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

9th Standard Science Chapter 5 Atomic Structure Important Question Paper Question Bank Software Jul-30 , 2019
Atomic Structure
Atomic Structure Important Questions
9th Standard
-
Reg.No. :
Science
Time :
01:00:00 Hrs
Total Marks :
50
-
Among the following the odd pair is
(a)8O18,9F19
(b)18Ar40,7N14
(c)14Si30, 15P31
(d)24Cr54, 19K39
-
The term nucleons refer to
(a)Protons and electrons
(b)only Neutrons
(c)electrons and neutrons
(d)Protons and neutrons
-
The correct electronic configuration of potassium is
(a)2,8,9
(b)2,8,1
(c)2,8,8,1
(d)2,8,8,3
-
Law of multiple proportions was proposed by _________
(a)J. Ritcher
(b)Rutherford
(c)John Dalton
(d)J.J Thomson
-
An alpha particle is identical with a ___________Nucleus.
(a)Argon
(b)Chlorine
(c)Neon
(d)Helium
-
Calcium and Argon are examples of a pair of __________
-
_________ isotope is used in the nuclear reactors.
-
The valency of Argon is ________
-
The law of reciprocal proportions was proposed by
-
Matter consists of very small and indivisible particles called________
-
__________has no neutron.
-
In an atom, electrons revolve around the nucleus in fixed orbits
(a) True(b) False -
Smaller the size of the orbit, lower is the energy of the orbit
(a) True(b) False -
The maximum number of electron in L Shell is 10
(a) True(b) False -
Atoms of an element combine in small whole numbers to form molecules.
(a) True(b) False -
Orbit is the path by which electrons revolve around the nucleus.
(a) True(b) False -
Dalton
-
Rutherford
-
Mass of proton
-
Law of multiple proportion·
-
Law of combining volumes
-
Name an element which has the same number of electrons in its first and second shell
-
Compare the charge and mass of protons and electrons.
-
Ca2+ has completely filled outer shell. Justify your answer
-
Arrange the following in the increasing order of atomic number
Calcium, Silicon, Boron, Magnesium, Oxygen, Helium, Neon, Sulphur, Fluorine and Sodium -
Copy the following and write the names of the laws and their simple definitions in the space provided.
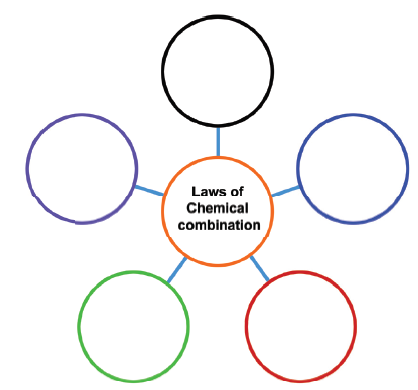
-
Methane burns in oxygen to form carbon dioxide and water vapour as given by the equation
CH4(g) + 2O2(g) ➝ CO2(g)+ 2H2O(g)
Calculate: (i) the volume of oxygen needed to burn completely 50 cm3 of methane and (ii) the volume of carbon dioxide formed in this case. -
List the uses of isotopes?
-
Draw the structure of oxygen and sulphur atoms.
-
Hydrogen sulphide (H2S) contains 94.11% sulphur, water (H2O) contains 11.11% hydrogen and sulphur dioxide (SO2) contains 50% of oxygen. Show that the results are in agreement with the law of reciprocal proportions
-
Explain the postulates of Bohr’s atomic model.
5 x 1 = 5
6 x 1 = 6
5 x 1 = 5
5 x 1 = 5
(1)
First atomic theory
(2)
Gay Lussac
(3)
1.67 x 10-24g
(4)
Discovery of nucleus
(5)
John Dalton
5 x 2 = 10
3 x 3 = 9
2 x 5 = 10






 9th Standard Science Syllabus
9th Standard Science Syllabus  9th Standard Science Study Materials
9th Standard Science Study Materials

Reviews & Comments about 9th Standard Science Chapter 5 Atomic Structure Important Question Paper
Write your Comment