CBSE 11th Standard Chemistry Subject Chemical Bonding and Molecular Structure Ncert Exemplar 3 Mark Questions With Solution 2021
By QB365
QB365 Provides the updated NCERT Exemplar Questions for Class
11, and also provide the detail solution for each and every NCERT Exemplar questions. NCERT Exemplar questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 11th Standard Chemistry Subject Chemical Bonding and Molecular Structure Ncert Exemplar 3 Mark Questions With Solution 2021
11th Standard CBSE
-
Reg.No. :
Chemistry
-
Write lewis structure of the following compounds and show formal charge each atom.
(a) -
Write the resonance structures for NO2
(a) -
What is meant by the term average bond enthalpy? Why is there difference in bond enthalpy of O - H bond in ethanol (C2H5OH) and water?
(a) -
Give reasons for the following.
Water molecule has bent structure whereas carbon dioxide molecule is linear.(a) -
Predict the hybridisation of each carbon in the molecule of organic compound given below. Also indicate the total number of \(\sigma \ and \ \pi \)-bonds in this molecule.
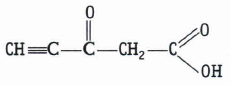 (a)
(a)




























