CBSE 12th Standard Chemistry Subject Aldehydes , Ketones and Carboxylic Acids Chapter Case Study Questions 2021
By QB365
QB365 Provides the updated CASE Study Questions for Class 12 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 12th Standard Chemistry Subject Aldehydes , Ketones and Carboxylic Acids Case Study Questions 2021
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Read the passage given below and answer the following questions:
(A), (B) and (C) are three non-cyclic functional isomers of a carbonyl compound with molecular formula C4H8O. Isomers (A) and (C) give positive Tollen's test whereas isomer (B) does not give Tollen's test but gives positive iodoform test. Isomers (A) and (B) on reduction with Zn(Hg)/conc. HCI give the same product (D).
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Compound A is
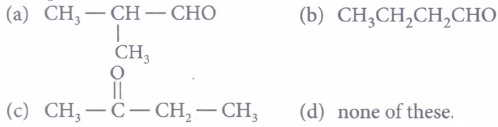
(ii) Compound (B) can be obtained by\({ (a) } \ \mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{C}-\mathrm{CH}_{2}-\mathrm{CH}_{3} \frac{\text { dil. } \mathrm{H}_{2} \mathrm{SO}_{4}+\mathrm{HgSO}_{4}}{333 \mathrm{~K}}\) \((b) \ \left(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COO}\right)_{2} \mathrm{Ca} \stackrel{\text { Dry distill }}{\longrightarrow}\) \((c) \ \mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{C}-\mathrm{CH}_{3} \frac{\mathrm{B}_{2} \mathrm{H}_{6} / \mathrm{THF}}{\mathrm{H}_{2} \mathrm{O}_{2} / \mathrm{NaOH}}\) \((d) \ \mathrm{CH}_{3}-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}_{3} \frac{\mathrm{O}_{3}}{\mathrm{Zn} / \mathrm{H}_{2} \mathrm{O}}\) (iii) Out of (A), (B) and (C) isomers, which one is least reactive towards addition of HCN ?
(a) A (b) B (c) C (d) All are equally reactive (iv) What will be the product when (B) reacts with ethylene glycol in presence ofHCI gas?
 (a)
(a) -
Read the passage given below and answer the following questions:
Aldehydes and ketones are reduced to primary and secondary alcohols respectively by NaBH4 or LiAIH4 as well as catalytic hydrogenation. The carbonyl group of aldehydes and ketones is reduced to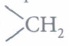 group on treatment with Zn-Hg and cone, HCI (Clemmensen reduction) or with hydrazine followed by NaOH or KOH in highly boiling solvent such, as ethylene glycol (Wolff- Kishner reduction).
group on treatment with Zn-Hg and cone, HCI (Clemmensen reduction) or with hydrazine followed by NaOH or KOH in highly boiling solvent such, as ethylene glycol (Wolff- Kishner reduction).
Aldehydes differ from ketones in their oxidation reactions. Aldehydes are easily oxidised to carboxylic acids on treatment with HNO3, KMnO4, K2Cr2O7 etc. Even mild oxidising agents mainly Tollens' reagent and Fehling's solution also oxidise aldehydes. Ketones are generally oxidised under vigorous conditions i.e., strong oxidising agents and at elevated temperatures, to give mixture of carboxylic acids having lesser number of C-atoms than the parent ketone.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Which of the following cannot be made by reduction of ketone or aldehyde with NaBH4 in methanol?(a) 1-Butanol (b) 2-Butanol (c) 2-Methyl-I-propanol (d) 2-Methyl-2-propanol (ii) The carbonyl compound producing an optically active product by reaction with LiAlH4 is
(a) propanone (b) butanone (c) 3-pentanone (d) benzophenone (iii) A substance C4H10O(X) yields on oxidation a compound C4H8O which gives an oxime and a positive iodoform test. The substance X on treatment with cone. H2S04 gives C4Hs. The structure of the compound (X) is
(a) CH3CH2CH2CH2OH (b) CH3CH(OH)CH2CH3 (c) (CH3)3COH (d) CH3CH2-O-CH2CH3 (iv) In the oxidation
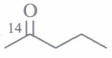 of by acidified K2Cr2O7, the products are
of by acidified K2Cr2O7, the products are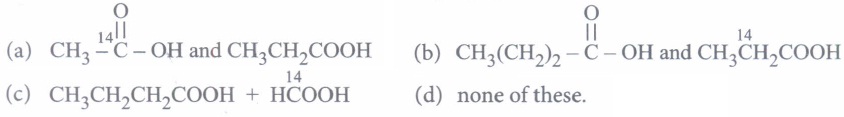 (a)
(a) -
Read the passage given below and answer the following questions:
Carboxylic acids having an a-hydrogen atom when treated with chlorine or bromine in the presence of small amount of red phosphorus gives a-halo carboxylic acids. The reaction is known as Hell- Volhard-Zelinsky reaction.
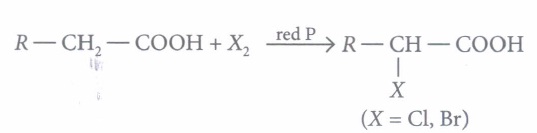
When sodium salt of carboxylic acid is heated with soda lime it loses carbon dioxide and gives hydrocarbon with less number of C-atoms.

In these questions (i - iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: (CH3)3CCOOH does not give H.V.Z reaction.
Reason: (CH3)3CCOOH does not have \(\alpha\)-hydrogen atom.
(ii) Assertion: H.V.Z. reaction involves the treatment of carboxylic acids having \(\alpha\)-hydrogens with Cl2 or Br2 in presence of small amount of redphosphorus.
Reason : Phosphorus reacts with halogens to form phosphorus trihalides.
(iii) Assertion: C6H5COCH2COOH undergoes decarboxylation easily than C6H5COCOOH.
Reason : C6H5COCH2COOH is a 13-ketoacid.
(iv) Assertion: On heating 3-methylbutanoic acid with soda lime, isobutane is obtained.
Reason: Soda lime is a mixture of NaOH + CaO in the ratio 3 : 1.(a) -
Read the passage given below and answer the following questions:
Fehling's reagent: Fehling's reagent is a mixture of two solutions. Fehling's solution A is aqueous copper sulphate solution. Fehling's solution B is alkaline sodium potassium tartarate (Rochelle salt).
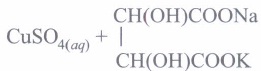
It is a mild oxidising agent. It is weaker than Tollens' reagent. It oxidises only aliphatic aldehydes to carboxylate ions and'itself gets reduced to reddish brown precipitate of cuprous oxide.
Aromatic aldehydes do not respond to Fehling's test. This reaction is used for the test of aliphatic aldehydes known as Fehling's reagent test.
In these questions ( i - iv) a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Fehling's solution can be used to distinguish between acetaldehyde and acetone.
Reason: Fehling's reagent is a mixture of two solutions.
(ii) Assertion: Aromatic aldehydes can be distinguished from aliphatic aldehydes by Fehling's solution.
Reason: Aromatic 'aldehydes reduce Fehling's solution, but aliphatic aldehydes do not.
(iii) Assertion: CH3CHO and C6H5CH2CHO cannot be distinguished chemically by Fehling's solution.
Reason: CH3CHO and C6H5CH2CHO can be distinguished by iodoform test.
(iv) Assertion: Formaldehyde, when heated with Fehling's reagent produces a reddish brown ppt. of Cu.
Reason: Fehling's reagent oxidises formaldehyde to formate ion.(a) -
Read the passage given below and answer the following questions:
Aldehydes and ketones undergo nucleophilic addition reactions .
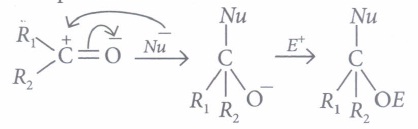
Carbonyl carbon is electron deficient hence acts as an electrophile. Nucleophile attacks on the electrophilic carbon atom of the carbonyl group from a direction perpendicular to the plane of the molecule.
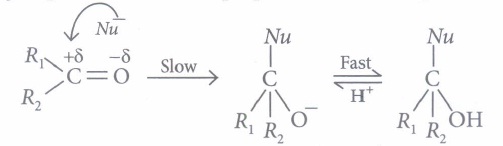
In this process, hybridisation of carbon atom changes from sp2 to sp3 and a tetrahedral alkoxide ion is formed as intermediate. This intermediate captures proton from the reaction medium to give the neutral product. Aldehydes are generally more reactive than ketones in nucleophilic addition reactions.
In these questions ( i - iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Benzaldehyde is more reactive than ethanal towards nucleophilic attack.
Reason: The overall effect of -land +R effect of phenyl group decreases the electron density on the carbon atom of >C=O group in benzaldehyde
(ii) Assertion: (CH3)3CCOC(CH3)3 and acetone can be distinguished by the reaction with NaHSO3.
Reason: HSO3 is the nucleophile in bisulphite addition.
(iii) Assertion: Ease of nucleophilic addition of the compounds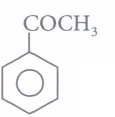 (I). CH3CHO(II) and CH3COCH3(III) is I > II > III.
(I). CH3CHO(II) and CH3COCH3(III) is I > II > III.
Reason : Aldehydes and ketones undergo nucleophilic addition reactions.
(iv) Assertion: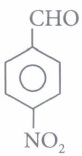 is more reactive towards nucleophilic addition reaction than
is more reactive towards nucleophilic addition reaction than 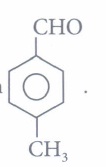
Reason : Reactivity of carbonyl group is due to electrophilic nature of carbonyl carbon.(a)































