CBSE 12th Standard Chemistry Subject Biomolecules Chapter Case Study Questions With Solution 2021
By QB365
QB365 Provides the updated CASE Study Questions for Class 12 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 12th Standard Chemistry Subject Biomolecules Case Study Questions With Solution 2021
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Read the passage given below and answer the following questions:
Pentose and hexose undergo intramolecular hemiacetal or hemiketal formation due to combination of the -OH group with the carbonyl group. The actual structure is either of five or six membered ring containing an oxygen atom. In the free state all pentoses and hexoses exist in pyranose form (resembling pyran). However, in the combined state some of them exist as five membered cyclic structures, called furanose (resembling furan).

The cyclic structure of glucose is represented by Haworth structure:
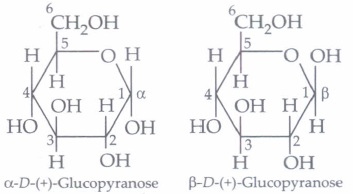
\(\alpha \) and \(\beta\) -D-glucose have different configuration at anomeric (C-l) carbon atom, hence are called anomers and the C-l carbon atom is called anomeric carbon (glycosidic carbon).
The six membered cyclic structure of glucose is called pyranose structure.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) \(\alpha\) -D(+)-glucose and \(\beta\) -D( +)glucose are(a) enantiomers (b) conformers (c) epimers (d) anomers (ii) The following carbohydrate is
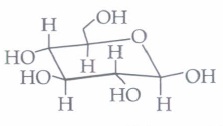
(a) a ketohexose (b) an aldohexose (c) an \(\alpha \)-furanose (d) an \(\alpha \)-pyranose (iii) In the following structure,
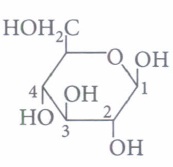
anomeric carbon is(a) C-l (b) C-2 (c) C-3 (d) C-4 (iv) The term anomers of glucose refers to
(a) isomers of glucose that differ in configurations at carbons one and four (C-l and C-4) (b) a mixture of (D)-glucose and (L)-glucose (c) enantiomers of glucose (d) isomers of glucose that differ in configuration at carbon one (C-l). (a) -
Read the passage given below and answer the following questions:
When a solution of an \(\alpha \) -amino acid is placed in an electric field depending on the pH of the medium, following three cases may happen.

(i) In alkaline solution,\(\alpha \) -amino acids exist as anion II, and there is a net migration of amino acid towards the anode.
(ii) In acidic solution, \(\alpha \) -amino acids exist as cation III, and there is a net migration of amino acid towards the cathode.
(iii) If II and III are exactly balanced there is no net migration; under such conditions anyone molecule exists as a positive ion and as a negative ion for exactly the same amount of time, and any small movement in the direction of one electrode is subsequently cancelled by an equal movement back toward the other electrode. The pH of the solution in which a particular amino acid does not migrate under the influence of an electric field is called the isoelectric point of that amino acid.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i)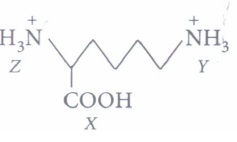
Arrange in order of increasing acid strengths.(a) X>Z>Y (b) Z (c) X>Y>Z (d) Z>X>Y (ii) In aqueous solutions, amino acids mostly exist as
\(\text { (a) } \mathrm{NH}_{2}-\mathrm{CH} R-\mathrm{COOH}\) \(\text { (b) } \mathrm{NH}_{2}-\mathrm{CH} R-\mathrm{COO}^{-}\) \(\text { (c) } \mathrm{NH}_{3} \mathrm{CH} R \mathrm{COOH}\) \(\text { (d) } \mathrm{H}_{3} \mathrm{NCH} R \mathrm{COO}^{-}\) (iii) Amino acids are least soluble
(a) at pH 1 (b) at pH 7 (c) at their isoelectric points (d) none of these. (iv) The \(pK_{a_{1}}\)and \(pK_{a_{2}}\) of an amino acid are 2.3 and 9.7 respectively. The isoelectric point of the amino acid is
(a) 12.0 (b) 7.4 (c) 6.0 (d) 3.7 (a) -
Read the passage given below and answer the following questions:
Carbohydrates can exist in either of two conformations, as determined by the orientation of the hydroxyl group about the asymmetric carbon farthest from the carbonyl.
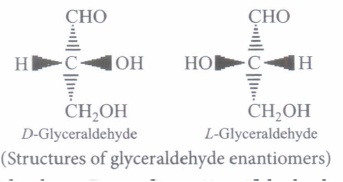
By convention, a monosaccharide is said to have D-configuration if the hydroxyl group attached to the asymmetric carbon atom adjacent to the - CH2OH group is on the right hand side irrespective of the positions of the other hydroxyl groups. On the other hand, the molecule is assigned L-configuration if the - OH group attached to the carbon adjacent to the - CH2OH group is on the left hand side.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) D-Glyceraldehyde and L-Glyc~raldehyde are(a) epimers (b) enantiomers (c) anomers (d) conformational diasteriomers (ii) Which of the following monosaccharides, is the majority found in the human body?
(a) D-type (b) L-type (c) Both of these (d) None of these (iii) Monosaccharides contain
(a) always six carbon atoms (b) always five carbon atoms (c) always four carbon atoms (d) may contain 3 to 7 carbon atoms (iv) The correct corresponding order of names of four aldoses with configuration given below
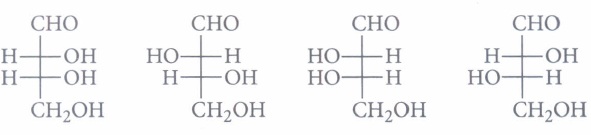
respectively, is(a) L-erythrose, L-threose, L-erythrose, D-threose (b) D-threose, D-erythrose, L-tl?repse, L-erythrose (c) L-erythrose, L-threose, D-erythrose, D-threose (d) D-erythrose, D-threose, L-erythrose, L-threose. (a) -
Read the passage given below and answer the following questions:
Carbohydrates are polyhydroxy aldehydes and ketones and those compounds which on hydrolysis give such compounds are also carbohydrates. The carbohydrates which are not hydrolysed are called monosaccharides. Monosaccharides with aldehydic group are called aldose and those which free ketonic groups are called ketose. Carbohydrates are optically active. Number of optical isomers = 2n
Where n = number of asymmetric carbons. Carbohydrates are mainly synthesised by plants during photosynthesis.
The monosaccharides give the characteristic reactions of alcohols and carbonyl group (aldehydes and ketones). It has been found that these monosaccharides exist in the form of cyclic structures. In cyclization, the -OH groups (generally C5 or C4 in aldohexoses and C5 or C6 in ketohexoses) combine with the aldehyde or keto group. As a result, cyclic structures of five or six membered rings containing one oxygen atom are formed, e.g., glucose forms a ring structure. Glucose contains one aldehyde group, one 1o alcoholic group and four 2o alcoholic groups in its open chain structure.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) First member of ketos sugar is(a) ketotriose (b) ketotetrose (c) ketopentose (d) ketohexose (ii) In CH2OHCHOHCHOHCHOHCHOHCHO, the number of optical isomers will be
(a) 16 (b) 8 (c) 32 (d) 4 (iii) Some statements are given below:
1. Glucose is aldohexose.
2. Naturally occurring glucose is dextrorotatory.
3. Glucose contains three, chiral centres.
4. Glucose contains one 1o alcoholic group and four 2o alcoholic groups.
Among the above, correct statements are(a) 1 and 2 only (b) 3 and 4 only (c) 1,2 and 4 only (d) 1,2,3 and 4 (iv) Which of the following reactions of glucose can be explained only by its cyclic structure?
(a) Glucose forms cyanohydrin with HCN (b) Glucose reacts with hydroxylamine to form an oxime (c) Pentaacetate of glucose does not react with hydroxylamine (d) Glucose is oxidised by nitric acid to gluconic acid . (a)































