









By QB365 on 31 Dec, 2022
QB365 provides a detailed and simple solution for every Possible Questions in Class 12 Chemistry Subject - Important 2 Mark English Medium. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
12th Standard
Chemistry
Answer all the following questions.
Give the uses of zinc.
The selection of reducing agent depends on the thermodynamic factor: Explain with an example.
Complete the following reactions.
a. \(B(OH)_3 + NH_3\longrightarrow \)
b. \(Na_{ 2 }B_{ 4 }{ O }_{ 7 }+{ { H }_{ 2 }{ SO }_{ 4 }+{ 5H }_{ 2 }O\longrightarrow }\)
c. \({ B }_{ 2 }{ H }_{ 6 }+2NaOH+2{ H }_{ 2 }O\longrightarrow \)
d. \({ B }_{ 2 }{ H }_{ 6 }+6{ CH }_{ 3 }OH\longrightarrow \)
e. \(4{ BF }_{ 3 }+3{ H }_{ 2 }O\longrightarrow \)
f. \(HCOOH+{ H }_{ 2 }{ SO }_{ 4 }\longrightarrow \)
g. \(2SiCl_{ 4 }+NH\)3
h. SiCl4 + 4C2H5OH \(\rightarrow\)
i. 2\(B+6NaOH\longrightarrow \)
j. \({ H }_{ 2 }{ B }_{ 4 }{ O }_{ 7 }\overset { Red\ hot }{ \rightarrow } \)
Give the uses of argon.
What are interstitial compounds?
In an octahedral crystal field, draw the figure to show splitting of d orbitals
Why ionic crystals are hard and brittle?
Consider the oxidation of nitric oxide to form NO2
2NO(g) + O2(g) ➝2NO2(g)
(a). Express the rate of the reaction in terms of changes in the concentration of NO,O2 and NO2.
(b). At a particular instant, when [O2] is decreasing at 0.2 mol L−1s−1 at what rate is [NO2] increasing at that instant?
Rate constant k of a reaction varies with temperature T according to the following Arrhenius equation \(\log K=\log A-\frac { { E }_{ a } }{ 2.303R } \left( \frac { 1 }{ T } \right) \)Where Ea is the activation energy. When a graph is plotted for log k Vs \(\frac{1}{T}\) a straight line with a slope of -4000K is obtained. Calculate the activation energy.
When aqueous ammonia is added to CuSO4 solution, the solution turns deep blue due to the formation of tetra ammine copper (II) complex,\({ [Cu({ H }_{ 2 }O)_4] }_{ (aq) }^{ 2+ }+ 4{ NH }_{ 3 }(aq)\rightleftharpoons { [Cu{ ({ NH }_{ 3 }) }_{ 4 }] }_{ (aq) }^{ 2+ }\) among H2O and NH3 Which is stronger Lewis base.
A solution of 0.10M of a weak electrolyte is found to be dissociated to the extent of 1.20% at 25oC. Find the dissociation constant of the acid.
Explain the function of H2 - O2 fuel cell.
The resistance of a conductivity cell is measured as 190 Ω using 0.1M KCl solution (specific conductance of 0.1M KCl is 1.3 Sm-1). When the same cell is filled with 0.003 M sodium chloride solution, the measured resistance is 6.3KΩ. Both these measurements are made at a particular temperature. Calculate the specific and molar conductance of NaCl solution.
What happens when a colloidal sol of Fe(OH)3 and As2S3 are mixed?
Comment on the statement: Colloid is not a substance but it is a state of substance.
Complete the following reactions
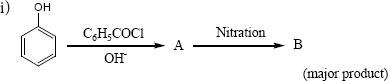
ii) \(C_6H_5-CH_{2}CH(OH)CH(CH_3)_2 \overset{ConH_2SO_4}\longrightarrow\)
How will you convert benzaldehyde into the following compounds?
(i) benzophenone
(ii) benzoic acid
(iii) α-hydroxyphenylaceticacid.
Complete the following reaction
Give two difference between Hormones and vitamins.
Write the structural formula of aspirin.
Name some reducing agents used to convert Metal oxides to metal
What is burnt alum?
Arrange the following as indicated below:
(i) F2, Cl2, Br2, I2 - increasing bond dissociation enthalpy.
(ii) HF, HCI, HBr, HI - increasing acidic strength.
Complete the following equations.
(i) 2 CrO42- + 2H+ ⟶
(ii) KMnO4 ⟶
Calculate the magnetic moment of [Fe(H2O)6]2+, if atomic number of Fe is 26.
Frenkel defect is not show by alkali metal halides but silver halides do. Give reason.
Define:- Half life period.
Define degree of dissociation.
What are secondary cells?
What is Tyndall effect?
Answers
(i) Metallic zinc is used in galvanising metals such as iron and steel structures to protect them from rusting and corrosion.
(ii) Brass an alloy of zinc is used in water valves and communication equipment as it is highly resistant to corrosion.
(iii) Zinc is also used to produce die-castings in the automobile, electrical and hardware industries.
(i) The extraction of metals from their oxides can be carried out by using different reducing agents.
(ii) Consider the following reaction
\(\frac{2}{\mathrm{y}} \mathrm{M}_{\mathrm{x}} \mathrm{O}_{\mathrm{y}(\mathrm{s})} \rightarrow \frac{2 \mathrm{x}}{\mathrm{y}} \mathrm{M}_{(s)}+\mathrm{O}_{ 2(\mathrm{~g})}\) (1)
(iii) The above reduction may be carried out with carbon. In this case the reducing agent carbon may be oxidized to either CO or CO2
\(\mathrm{C}+\mathrm{O}_{2} \rightarrow \mathrm{CO}_{2(\mathrm{~g})} \) (2)
\(2 \mathrm{C}+\mathrm{O}_{2} \rightarrow 2 \mathrm{CO}_{(\mathrm{g})} \) (3)
(iv) If CO is used as a reducing agent
\(2 \mathrm{CO}+\mathrm{O}_{2} \rightarrow 2 \mathrm{CO}_{2(\mathrm{~g})}\) (4)
(v) A suitable reducing agent is selected based on the thermodynamics considerations.
(vi) We know that for a spontaneous reaction, the change in free energy (\(\triangle\)G) should be negative.
(vii) Therefore, thermodynamically, the reduction of metal oxide with a given reducing agent can occur if the free energy change for the coupled reaction is negative.
(viii) Hence, the reducing agent is selected in such a way that it provides a large negative \(\triangle\)G value for the coupled reaction.
(a) B(OH)3 + NH3\(\overset { \Delta }{ \longrightarrow } \) BN + 3H2O
(Boron nitride)
(b) Na2B4O7 + H2SO4 + 5H2O \(\longrightarrow \) 4H3BO3 + Na2SO4
(Boric acid)
(c) B2H6 + 2NaOH + 2H2O \(\longrightarrow \)2NaBO2 + 6H2
(Sodium metaborate)
(d) B2H6 + 6CH3OH \(\longrightarrow \)2B(OCH3)3 + 6H2O
(Trimethyl borate)
(e) 4BF3 + 3H2O \(\longrightarrow \) H3BO3 + 3H+ + 3[BF4]-
(Boric acid)
(f) HCOOH + H2SO4 \(\longrightarrow \)CO + H2SO4. H2O
(Carbon monoxide)
(g) 2SiCl4 + NH3 \(\overset { 330K }{ \underset { ether }{ \longrightarrow } } \)Cl3Si- NH - SiCl3 + 2HCl
(Chlorosilazane)
(h) SiCl4 + 4C2H5OH\(\longrightarrow \)Si(OC2H5)4 + 4HCI
(Tetraethoxysilane)
(i) 2B + 6NaOH\(\longrightarrow \) 2Na3BO3 + 3H2
(j) H2B4O7 \(\xrightarrow[]{Redhot}\) 2B2O3 + H2O
Argon prevents the oxidation of hot filament and prolongs the life in filament bulbs.
An interstitial compound or alloy is a compound that is formed when small atoms like hydrogen, boron, carbon or nitrogen are trapped in the interstitial holes in a metal lattice. They are usually non-stoichiometric compounds. Transition metals form a number of interstitial compounds such as TiC, ZrH1.92, Mn4N etc.
Properties of interstitial compound
(i) They are hard and show electrical and thermal conductivity.
(ii) They have high melting points higher than those of pure metals.
(iii) Transition metal hydrides are used as powerful reducing agents
(iv) Metallic carbides are chemically inert.
The energy of the two eg orbitals will increase by \(\frac{3}{5} \Delta_{O}\) and that of the three t2g will decrease by (2/5) \(\Delta_{O}\)
The structural units of an ionic crystal are cations and anions. They are bound together by strong electrostatic attractive forces. To maximize the attractive force, cations are surrounded by as many anions as possible and vice versa. Hence they are hard and brittle.
a) \(Rate=\frac { -1 }{ 2 } \frac { d[NO] }{ dt } =\frac { -d[{ O }_{ 2 }] }{ dt } =\frac { 1 }{ 2 } \frac { d\left[ { NO }_{ 2 } \right] }{ dt } \)
b) \(\frac { -d\left[ { O }_{ 2 } \right] }{ dt } =\frac { 1 }{ 2 } \frac { d\left[ { NO }_{ 2 } \right] }{ dt } \)
\(\frac { d[{ NO }_{ 2 }] }{ dt } =2 \times\left( \frac { -d\left[ { O }_{ 2 } \right] }{ dt } \right) =2\times 0.2 \ { mol \ L }^{ -1 }{ s }^{ -1 }\)
= 0.4 mol L-1s-1
\(\log K=\log A-\frac { { E }_{ a } }{ 2.303R } \left( \frac { 1 }{ T } \right) \)
y = c + mx
\(m=-\frac { { E }_{ a } }{ 2.303R } \)
Ea = -2.303 Rm
Ea = -2.303 x 8.314 x (-4000)
Ea = 76,589J mol-1
Ea = 76.589 KJ mol-1
(i) According to Lewis theory a species that donates a pair of electron is called Lewis base.
(ii) Nitrogen more in NH3 is less electro negative than oxygen in water. So the non - bonded electron pair on nitrogen is more available for sharing than a non - bonded electron pair on oxygen atom. So NH3 is a stronger lewis base than H2O.
Given that \(\alpha=1.20\)%=\(\frac{1.20}{100}\times1.2\times10^{-2}\)
\(K_{a}=\alpha^{2}c\)
\(=(1.2\times10^{-2})^{2}(0.1)=1.44\times10^{-4}\times10^{-1}\)
=\(1.44\times10^{-5}\)
(i) In this case, hydrogen act as a fuel and oxygen as an oxidant and the electrolyte is aqueous KOH maintained at 200oC and 20-40 atm. Porous graphite electrode containing Ni and NiO serves as the inert electrodes.
(ii) Hydrogen and oxygen gases are bubbled through the anode and cathode, respectively.
Oxidation occurs at the anode:
\(2 \mathrm{H}_{2(\mathrm{~g})}+4 \mathrm{OH}_{(a q)}^{-} \rightarrow 4 \mathrm{H}_{2} \mathrm{O}_{(l)}+4 \mathrm{e}^{-}\)
Reduction occurs at the cathode:
\(\mathrm{O}_{2(\mathrm{~g})}+2 \mathrm{H}_{2} \mathrm{O}_{(t)}+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}_{(\mathrm{aq})}^{-}\)
(iii) The overall reaction is \(2 \mathrm{H}_{2(\mathrm{~g})}+\mathrm{O}_{2(\mathrm{~g})} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}_{(1)}\)
(iv) The above reaction is the same as the hydrogen combustion reaction, however, they do not react directly ie., the oxidation and reduction reactions take place separately at the anode and cathode respectively like H2-O2 fuel cell. Other fuel cells like propane -O2 and methane O2 have also been developed.
Given that
κ = 1.3 Sm-1 (for 0.1M KCl solution)
R = 190 Ω
\(\kappa = \frac{1}{R}(\frac{l}{A})\)
κ . R =\((\frac{l}{A})\) = (1.3 Sm-1) (190Ω)
= 247 m-1
\(\kappa_{(NaCl)} = \frac{1}{R_{(NaCl)}} (\frac{l}{A})\)
\(= \frac{1}{6.3 K\Omega}(247 m^{-1})\) (6.3KΩ = 6.3 x 103Ω)
= 39.2 x 10-3 Sm-1
\(\Lambda_m = \frac{\kappa \times 10^{-3} mol^{-1} m^3}{M}\)
\(=\frac{39.2 \times 10 ^{-3}(Sm^{-1})10^-3 (mol^{-1} m^3)}{0.003}\)
\(\Lambda_m\) = 13.04 \(\times\) 10-3 Sm2 mol-1
(i) Neutralisation of chargers of ion will taken place and hence precipitation will take place (ie) Fe3+ and S2- ion changes are neutralized. No new compounds are formed.
(ii) Fe(OH)3 is a positive Sol
(iii) As2S3 is a negative Sol
(i) A Colloid depends on the size of the particle. A Colloid is formed when the size of the particle lies between 1 nm and 100 nm. For example soap dissolves in water to form colloidal soap solution whereas it dissolves in alcohol to form a true solution. Thus change of state takes place. A colloidal state maybe an intermediate between a true solution and a suspension.
(ii) Also some crystalloids under certain conditions can be colloids. NaCl is a crystalloid in aqueous medium; but when mixed with benzene it acts as a colloid.
(i)
n-Nitro benzoate (Major Product)
(ii)
(ii) benzoic acid
(iii) α - hydroxyphenylaceticacid.
| Hormone | Vitamin |
|---|---|
| Synthesized in animal bodies | Synthesised in plants |
| Produced in ductless (endocrine glands) | Have to be supplied in diet except (Vitamin D) |
| These are not stored in body but are continuously produced | These remain stored in the body to keep away diseases |
| Eg: Androgen, Estrogen, thyroxine etc. | Eg: Vitamin A (Retinol) Vitamin C (Ascorbic acid) |
Aspirin is o-acetyl salicylic acid
Metal oxides to metal : Carbon, carbon monoxide, hydrogen, aluminum and metals like sodium.
On heating to 475 K potash alum loses water of hydration and swells up. The swollen mass is known as burnt alum.
(i) l2 < F2 < Br2 < Cl2·
(ii) HF < Hq < HBr < HI.
(i) 2 CrO42- + 2H+ ⟶ Cr2O72- + H2O
(ii) 2KMnO4 \(\overset { \triangle }{ \underset { 513 k }{ \longrightarrow } } \) K2Mn4O4 + MnO2 + O2
Magnetic moment (μ) = \(\sqrt { n(n+2) } \) BM
Fe (z = 26) = 1s2 2s2 2p6 3s2 3p6 4s2 3d6
Fe2+ = 1s2 2s2 2p6 3s2 3p6 3d6 4s0
∴μ = \(\sqrt { n(n+2) } \) = \(\sqrt { 4(4+2) } =\sqrt { 24 } \)
= 4. 89 BM
Frenkel defect occurs in a Silver halides due to small size of Ag+ ions which occupy interstitial position. Whereas in alkali metal halides, size of the cation and anion are almost similar so alkali metal ions do not fit into the interstitial sites.
The half life of a reaction is defined as the time required for the reactant concentration to reach one half its initial value.
Degree of dissociation (α) is the fraction of the total number of moles of a substance that dissociates at equilibrium.
\(\alpha=\frac{\text { Number of moles dissociated }}{\text { Total number of moles }}\)
Secondary cells are those which can be recharged by passing electric current through them and hence can be used over and again.
(i) When a strong beam of light is passed through a sol, the path of light is illuminated by the scattering of light by colloidal particles.
(ii) The phenomenon of the scattering of light by the sol particles is called Tyn all effect.











