









By QB365 on 31 Dec, 2022
QB365 provides a detailed and simple solution for every Possible Questions in Class 12 Chemistry Subject - Important 5 Mark English Medium. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
12th Standard
Chemistry
Answer all the following questions.
Using the Ellingham diagram,
(A) Predict the conditions under which
(i) Aluminium might be expected to reduce magnesia.
(ii) Magnesium could reduce alumina.
(B) it is possible to reduce Fe2O3 by coke at a temperature around 1200K
Give the limitations of Ellingham diagram.
Give the balanced equation for the reaction between chlorine with cold NaOH and hot NaOH.
Write the postulates of Werner’s theory.
On the basis of VB theory explain the nature of bonding in [Co(C2O4)3]3-
Differentiate crystalline solids and amorphous solids.
Calculate the percentage efficiency of packing in case of body centered cubic crystal.
What is an elementary reaction? Give the differences between order and molecularity of a reaction.
Discuss the Lowry – Bronsted concept of acids and bases.
State Kohlrausch Law. How is it useful to determine the molar conductivity of weak electrolyte at infinite dilution.
What is the difference between homogenous and hetrogenous catalysis?
Describe adsorption theory of catalysis
Predict the product A,B,X and Y in the following sequence of reaction
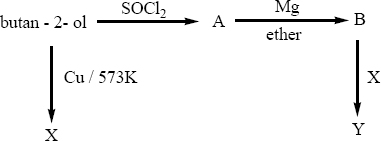
3,3 – dimethylbutan -2-ol on treatment with conc. H 2SO4 to give tetramethyl ethylene as a major product. Suggest a suitable mechanism.
Identify A, B, C and D
\(\text { ethanoic acid } \stackrel{\mathrm{SOCl}_{2}}{\longrightarrow} \mathrm{A} \stackrel{\mathrm{Pd} / \mathrm{BaSO}_{4}}{\longrightarrow} \mathrm{B} \stackrel{\mathrm{NaOH}}{\longrightarrow} \mathrm{C} \stackrel {\longrightarrow}{\triangle} \mathrm{D}\)
An alkene (A) on ozonolysis gives propanone and aldehyde (B). When (B) is oxidised (C) is obtained. (C) is treated with Br2/P gives (D) which on hydrolysis gives (E). When propanone is treated with HCN followed by hydrolysis gives (E). Identify A, B, C, D and E.
Predict A,B,C and D for the following reaction
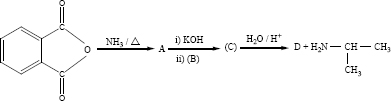
Identify A to E in the following sequence of reactions
\(\overset { {CH_3} {CL} }{ \underset { {AlCl}_3 }{ \longrightarrow } }\) A \(\overset { {HNO_3}/ {H_2So_4} }{ \underset { {} }{ \longrightarrow } }\) B \(\overset { {Sn} /{HCL} }{ \underset {{} }{ \longrightarrow } }\) (C) \(\overset { {NaNo_2}/ {HCL} }{ \underset { {O^o}C }{ \longrightarrow } }\) D \(\overset { {CuCN} }{ \underset { {}}{ \longrightarrow } }\) E
What are bio degradable polymers? Give examples.
How is terylene prepared?
Explain the preparation, properties, structure and uses of Diborane.
Complete the following reactions
(i) KCIO3\(\longrightarrow \)?
(ii) ZnS + O2\(\longrightarrow \) ?
(iii) Al2O3 + NaOH + H2O\(\longrightarrow \) ?
(iv) NaOH + SO2\(\longrightarrow \) ?
(v) KCI+ H2SO4 \(\longrightarrow \) ?
Complete the following equations:
a. 4NaCI + MnO2 + 4H2SO4 \(\longrightarrow \)?
b. 6XeF4 + 12H2O\(\longrightarrow \) ?
(i) \(\mathrm{PCl}_{5}+\mathrm{Ag} \longrightarrow \text { ? } \)
(ii) \(\mathrm{AgCl}_{(s)}+\mathrm{NH}_{3(\mathrm{aq})} \longrightarrow ? \)
(iii) \(\mathrm{PCl}_{3} \stackrel{\text { Moist air }}{\longrightarrow} \text { ? } \)
(iv) \(\mathrm{PCl}_{5} \stackrel{\text { Moist air }}{\longrightarrow} ? \)
(v) \(\mathrm{NaH}_{2} \mathrm{PO}_{2}+\mathrm{HCl} \longrightarrow ?\)
Why is there a variation of atomic and ionic size as we move from Sc to Zn?
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers.
(i) K[Cr(H2O)2 (C2O4)2]
(ii) [Co(en)3]CI3
(iii) [Co(NH3)5(NO2)](NO3)2
(iv) [Pt (NH3)(H2O)CI2]
Write note on impurity defect?
If 30% of a first order reaction is completed in 12 mins, what percentage will be completed in 65.33 mins?
Calculate the pH of solution with HO+ concentrations in mol dm-3.
(i) 10-4
(ii) 10-7
(iii) 6.8 x 10-3
(iv) 3.2 x 10-5
(v) 0.035
(vi) 0.25
(vii) 5.4 x 10-9
(viii) 7.1 x 10-7
The standard reduction potential for the reaction Sn4+ + 2e- ⟶ Sn2+ is + 0.15v. Calcuate the free energy change of the reaction.
Answers
a) i) Ellingham diagram for the formation of Al2O3 and MgO intersects around 1500oC. Above this temp Mg lies above the Aluminium line. Hence only above 1500oC Aluminium might be excepted to reduce magnesia.
ii) Ellingham diagram for the formation of MgO lies below the formation of Al2O3. Hence MgO is more stable than Al2O3. Hence Magnesium could reduce Alumina.
1. Below 983K, formation of CO line lies below many of the metal oxide formation in Ellingham diagram, hence CO is more effective reducing agent than Carbon.
2. But above this temperature Carbon lies below other metal oxides.
b) Around 1200K Carbon lies below the formation of Fe2O3. Hence it is possible to reduce Fe2O3 by coke at 1200K.
(i) Ellingham diagram is constructed based only on thermodynamic considerations. It gives information about the thermodynamic feasibility of a reaction. It does not tell anything about the rate of the reaction. More over, it does not give any idea about the possibility of other reactions that might be taking place.
(ii) The interpretation of \(\triangle\)G is based on the assumption that the reactants are in equilibrium with the product which is not always true.
Chlorine reacts with cold dilute alkali to give chloride and hypochlorite, while with hot concentrated alkali chlorides and chlorates are formed.
\(\mathrm{Cl}_{2}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{HCl}+\underset{\text { Hypochlorous acid }}{\mathrm{HOCl}} \)
\(\mathrm{HCl}+\mathrm{NaOH} \rightarrow \mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O} \)
\(\mathrm{HOCl}+\mathrm{NaOH} \rightarrow \mathrm{NaOCl}+\mathrm{H}_{2} \mathrm{O} \)
(Sodium hypo chlorite)
Overall Reaction
3Cl2 + 6NaOH \(\rightarrow\) NaClO3 + 5NaCl + 3H2O
(Sodium Chlorate)
Most of the elements exhibit, two types of valence namely primary valence and secondary valence and each element tend to satisfy both the valences.
The primary valence is referred the oxidation state of the metal atom.
The secondary valence as the coordination number. For example, according to Werner, the primary and secondary valences of cobalt are 3 and 6 respectively.
The primary valence of a metal ions ae always satisfied by negative ions.
For example in the complex CoCI3.6NH3. The primary valence of Co is +3 and is satisfied by 3CI- ions.
The secondary valence is satisfied by negative ions, neutral molecules, positive ions or the combination of these.
For example, in CoCl3.6NH3 complex primary valence of cobalt +3 and it is satisfied by 3 CI-.
The secondary valence of cobalt is 6 and is satisfied by six neutral ammonia molecules. where as in CoCI6.NH3.
Secondary valence of Co = 5{It is satisfied five neutral molecules and a Cl- ion}
According to Werner, there are two spheres of attraction around a metal atom/ion in a complex.
The inner /coordination sphere:
The groups present in this sphere are firmly attached to the metal.
The outer sphere / ionisation sphere:
The groups present in this sphere are loosely bound to the central metal ion and hence can be separated into ions upon dissolving the complex in a suitable solvent.
The primary valencies are non-directional. while the secondary valencies are directional.
The geometry of the complex is determined by the special arrangement of the groups which satisfy the secondary valence.
| Secondary valence | Geometry |
| 4 | Tetrahedral / Square planar |
| 6 | Octahedral |
In \(\left[\mathrm{Co}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]^{3-}\) Cobalt is in +3 oxidation state
\(\mathrm{Co}=[\mathrm{Ar}] 3 \mathrm{~d}^{7} 4 \mathrm{~s}^{2} \)
\(\mathrm{Co}^{3+}=3 \mathrm{~d}^{6} 4 \mathrm{~s}^{\circ}\)
It is diamagnetic; n = 0
d2sp3 hybridisation; μs = 0
| S. No | Crystalline Solids | Amorphous Solids |
| 1. | Long range orderly arrangement of constituents. | Short range, random arrangement of constituents. |
| 2. | Definite shape | Irregular shape |
| 3. | Anisotropic in nature | They are "isotropic" like liquids |
| 4. | They are true solids | They are considered as pseudo solids (or) super cooled liquids |
| 5. | Definite Heat of fusion | Heat of fusion is not definite |
| 6. | They have sharp melting points. | Gradually soften over a range of temperature and so can be moulded. |
| 7. | Eg: NaCl, diamond etc. | Eg: Rubber, plastics, glass etc. |
In bcc unit cell, ΔABC
AC2 = AB2 + BC2
\(AC=\sqrt { { AB }^{ 2 }+{ BC }^{ 2 } } \)
\(\\ AC=\sqrt { { a }^{ 2 }+{ a }^{ 2 } } =\sqrt { { 2a }^{ 2 } } =\sqrt { 2 } a\)
In ΔACG
AG2 = AC2 + CG2
\(AG=\sqrt { { AC }^{ 2 }+{ CG }^{ 2 } } \)
\(AG=\sqrt { { \left( \sqrt { 2a } \right) }^{ 2 }+{ a }^{ 2 } } \)
\(AG=\sqrt { { 2a }^{ 2 }+{ a }^{ 2 } } =\sqrt { { 3a }^{ 2 } } \)
\(AG=\sqrt { 3a } \)
\(\sqrt { 3 } a=4r\)
\(r=\frac { \sqrt { 3 } }{ 4 } a\)
∴ Volume of the sphere with radius 'r' \(=\frac { 4 }{ 3 } { \pi r }^{ 3 }\)
\(=\frac{4}{3}\pi { \left( \frac { \sqrt { 3 } }{ 4 } a \right) }^{ 3 }\)\(=\frac { \sqrt { 3 } }{ 16 } \pi { a }^{ 3 }\)
Number of spheres belong to a unit cell in BCC arrangement is equal to two and hence the total volume of all spheres.
(i) Packing fraction = \(=\frac{Total \quad volume \quad occupied \quad by \quad spheres \quad in \quad a \quad unit \quad cell}{volume \quad of \quad the \quad unit \quad cell}\times100\)
\(\therefore\)Volume of all spheres \(=2\times \left( \frac { \sqrt { 3 } \pi { a }^{ 3 } }{ 16 } \right) =\frac { \sqrt { 3 } \pi { a }^{ 3 } }{ 8 } \)
Packing fraction \(=\frac { \left( \frac { \sqrt { 3 } \pi { a }^{ 3 } }{ 8 } \right) }{ ({ a }^{ 3 }) } \times 100\)
\(=\frac { \sqrt { 3 } \pi }{ 8 } \times 100\)
\(\\ =\sqrt { 3 } \pi \times 12.5\)
= 1.732 x 3.14 x 12.5
= 68%
(a) Elementary reaction
Each and Every single step in a reaction mechanism is called an elementary reaction.
Rate = k[A] [B]
(b)
| Order of reaction | Molecularity of a reaction |
|---|---|
| Order of reaction is the sum of the powers of concentration terms involved in the experimentally determined rate law. | Molecularity of a reaction is the total number of reactant species that are involved in an elementary step. |
| It can be zero (or) fractional (or) integer | It is always a whole number, cannot be zero or a fractional number. |
| It is assigned for a overall reaction. | It is assigned for each elementary step of the mechanism. |
(i) An acid is defined as a substance that has a tendency to donate a proton to another substance and base is a substance that has a tendency to accept a proton form other substance.
(ii) In other words, an acid is a proton donor and a base is a proton acceptor.
(iii) When hydrogen chloride is dissolved in water, it donates a proton to the later. Thus, HCI behaves as an acid and H2O is base. The proton transfer from the acid to base can be represented as
HCI + H2O ⇌ H3O+ + Cl-
(iv) When ammonia is dissolved in water, it accepts a proton from water. In this case, ammonia (NH3) acts as a base and H2O is acid. The reaction is represented as
H2O + NH3 ⇌ NH4+ + OH-
(v) Let us consider the reverse reaction following equilibrium.
\(\underset { proton\ donar\\ \quad \quad \ (acid) }{ HCl } +\underset { Proton\ acceptor\\ \quad \quad \quad \quad (base) }{ { H }_{ 2 }O } \leftrightharpoons \underset { Proton\ donar\\ \quad \quad \quad \quad \ (acid) }{ { H }_{ 2 }{ O }^{ + } } +\underset { Proton\ acceptor\\ \quad \quad \quad \quad \ (base) }{ { Cl }^{ - } } \)
H3O+ donates a proton to Cl- to form HCI i.e., the products also behave as acid and base.
(vi) In general, Lowry - Bronsted (acid - base) reaction is represented as
Acid1 + Base2 ⇌ Acid2 + Base1
(vii) The species that remains after the donation of a proton is a base (Base1) and is called the conjugate base of the Bronsted acid (Acid1). In other words, chemical species that differ only by a proton are called conjugate acid - base pairs.
Kohlraush's law:
(i) At infinite dilution, the limiting molar conductivity of an electrolyte is equal to the sum of the limiting molar conductivities of its constituent ions. i.e., the molar conductivity is due to the independent migration of cations in one direction and anions in the opposite direction.
(ii) For a uni - univalent electrolyte such as NaCl, the Kohlraush's law is expressed as
\(\left(\Lambda_{\mathrm{m}}^{0}\right)_{\mathrm{NaCl}}=\left(\lambda_{\mathrm{m}}^{0}\right)_{\mathrm{Na}^{+}}+\left(\lambda_{\mathrm{m}}^{0}\right)_{\mathrm{Cl}}^{-}\)
(iii) In general, according to Kohlraush's law, the molar conductivity at infinite dilution for a electrolyte represented by the formula Ax By, is given below.
\(\left(\Lambda_{m}^{0}\right)_{A_{x} B_{y}}=x\left(\lambda_{m}^{0}\right)_{A^{y+}}+y\left(\lambda_{m}^{0}\right)_{B^{x-}}\)
b) Calculation of molar conductance at infinite dilution for weak electrolytes experimentally.
(i) However, the same can be calculated using Kohlraush's Law. For example, the molar conductance of CH3COOH, can be calculated using the experimentally determined molar conductivities of strong electrolytes HCl, NaCl and CH3COONa.
\(\Lambda_{\mathrm{CH}_{3} \mathrm{COONa}}^{0}=\lambda_{\mathrm{Na}^{+}}^{0}+\lambda_{\mathrm{CH}_{3} \mathrm{COO}^{-}}^{0} \) ..........(1)
\(\Lambda_{\mathrm{HCl}}^{0}=\lambda_{\mathrm{H}+}^{0}+\lambda_{\mathrm{Cl}^{-}}^{0} \) .........(2)
\(\Lambda_{\mathrm{NaCl}}^{0}=\lambda_{\mathrm{Na}^{+}}^{0}+\lambda_{\mathrm{Cl}^{-}}^{0}\) ...........(3)
(ii) Equation (1) + Equation (2) - Equation (3) gives,
\(\left(\Lambda_{\mathrm{CH}_{3} \mathrm{COONa}}^{0}\right)+\left(\Lambda_{\mathrm{HCl}}^{0}\right)-\left(\Lambda_{\mathrm{NaCl}}^{0}\right)=\lambda_{\mathrm{H}+}^{0}+\lambda_{\mathrm{CH}_{3} \mathrm{COO}-}^{0} \)
\(=\Lambda_{\mathrm{CH}_{2} \mathrm{COOH}}^{0}\)
| Homogenous catalysis | Heterogeneous catalysis |
|---|---|
| 1. In a catalysed reaction, the reactants, products and catalyst are present in the same phase. Ex: \( 2\mathrm{SO}_{2(\mathrm{~g})}+\mathrm{O}_{2(\mathrm{~g})}+[\mathrm{NO}]_{(\mathrm{g})} \rightarrow 2 \mathrm{SO}_{3(\mathrm{~g})}+[\mathrm{NO}]_{(\mathrm{g})} \) [NO] - catalyst; gaseous state SO2, O2 & SO3 are gases. |
1. In a catalysed reaction, the catalyst is present in a different phase (ie) it is not present in the same phase as that of the reactants or products. Ex: \(\mathrm{N}_{2(\mathrm{~g})}+3 \mathrm{H}_{2(\mathrm{~g})} \stackrel{\mathrm{Fe}_{(\mathrm{s})}}{\longrightarrow} 2 \mathrm{NH}_{3(\mathrm{~g})}\) Fe - catalyst; solid N2, H2 & NH3 are gases. |
| 2. It is not a contact catalysis. | 2. It is a contact catalysis and the mental catalyst will be in finely divided metal or as gauze. |
| 3. It is explained by intermediate compound formation theory. |
3. It is explained by adsorption theory. |
1. Langmuir explained the action of catalyst in heterogeneous catalysed reactions based on adsorption. The reactant molecules are adsorbed on the catalyst surfaces, so this can also be called as contact catalysis.
2. According to this theory, various steps involved in a heterogeneous catalyse reaction are given as follows:
(i) Reactant molecules diffuse from bulk to the catalyst surface.
(ii) The reactant molecules are adsorbed on the surface of the catalyst.
(iii) The adsorbed reactant molecules are activated and form activated complex which is decomposed to form the products.
(iv) The product molecules are desorbed.
(v) The product diffuse away from the surface of the catalyst.
Mechanism:
This mechanism undergoes Saytzeff rule.
During intramolecular dehydration, if there is a possibility to form a carbon - carbon double bond at different locations, the preferred location is the one that gives the more (highly) substituted alkene i.e., the stable alkene.
| Compound | Name |
| A | Acetylchloride |
| B | Acetaldehyde |
| C | 3 - Hydroxy butanal |
| D | Crotonaldehyde |
1. The materials that are readily decomposed by microorganisms in the environment are called biodegradable.
2. Natural polymers degrade on their own after certain period of time but the synthetic polymers do not.
3. It leads to serious environmental pollution. One of the solution to this problem is to produce biodegradable polymers which can be broken down by soil micro organism.
Examples:
(i) Polyhydroxy butyrate (PHB)
(ii) Polyhydroxy butyrate-co-A- hydroxyl valerate (PHBV)
(iii) Polyglycolic acid (PGA), Polylactic acid (PLA)
(iv) Poly ( E caprolactone) (PCL)
(v) Biodegradable polymers are used in medical field such as surgical sutures, plasma substitute etc...
4. these polymers are decomposed by enzyme action and are either metabolized or excreted from the body.
(i) Monomers :Ethylene glycol and terepathalic acid (or) dimethyl terephthalate.
(ii) Catalyst : Zinc acetate and antimony trioxide.
(iii) Temperature : 500 K
(iv) Product : Terylene
(v) Uses : blending with cotton or wool fibres and as glass reinforcing materials in safety helmets
Preparation:
As discussed earlier diborane can be prepared by the action of metal hydride with boron. This method is used for the industrial production.
Diborane can also be obtained in small quantities by the reaction of iodine with sodium borohydride in diglyme.
2NaBH4 + I2 ⟶ B2H6 + 2NaI + H2
On heating magnesium boride with HCl a mixture of volatile boranes are obtained.
2Mg3B2 + 12HCl ⟶ 6MgCl2 + B4H10 + H2
B4H10 + H2 ⟶ 2B2H6
(i) KCIO3 \(\overset { \Delta }{ \underset { { MnO }_{ 2 } }{ \longrightarrow } } \) 2KCI + 3O2
(ii) 2 ZnS + 3O2\(\longrightarrow \) 2 ZnO + 2 SO2
(iii) AIzO3(s) + 6 NaOH(aq) + 3 H2O(l) \(\longrightarrow \) 2 Na3[AI(OH)6](aq)
(iv) 2NaOH + SO2\(\longrightarrow \)Na2SO3 + H2O
(v) 2KCl + H2SO4 \(\longrightarrow \) 2 HCl + K2SO4
(a) NaCl + MnO2 + 4H2SO4 \(\longrightarrow \) MnCl2+ 4NaHSO4 + 2H2O + Cl2
(b) 6XeF4 + 12H2O \(\longrightarrow \) 4Xe + 2XeO3 + 24HF + 3O2
(i) PCl5 + 2Ag \(\longrightarrow \) 2 AgCl + PCl3
(ii) AgCI(s)+ 2NH3(aq) \(\longrightarrow \)[Ag(NH3)2]CI(aq)
(iii) PCl3 + 3H2O \(\longrightarrow \) H3PO3 + 3HCI
(iv) PCl5 + H2O \(\longrightarrow \) POCl3 + 2HCI
(v) NaH2PO2 + HCI \(\longrightarrow \) H3PO2 + NaCl
(i) It is generally expected a steady decrease in atomic radius along a period as the nuclear charge increases and the extra electrons are added to the same sub shell.
(ii) But for the 3d transition elements, the expected decrease in atomic radius is observed from Sc to V, thereafter up to Cu the atomic radius nearly remains the same.
(iii) As we move from Sc to Zn in 3d series the extra electrons are added to the 3d orbitals, the added 3d electrons only partially shield the increased nuclear charge and hence the effective nuclear charge increases slightly.
(iv) However, the extra electrons added to the 3d sub shell strongly repel the 4s electrons and these two forces are operated in opposite direction and as they tend to balance each other, it leads to constancy in atomic radii.
(v) At the end of the series, d - orbitals of Zinc contain 10 electrons in which the repulsive interaction between the electrons is more than the effective nuclear charge and hence, the orbitals slightly expand and atomic radius slightly increases.
(i) It exhibits both geometrical and optical isomerism
(a) Geometrical isomers:
(b) Optical isomers:
(ii) It shows two optical isomers
(iii) Ionisation isomers
Linkage isomers
[Co(NH3)5(NO2)(NO3)2], [CO(NH3)5 (ONO)](NO3)2
(iv) Geometrical isomers
(i) The defects in ionic solids is by adding impurity ions.
(ii) If the impurity ions are in different valance state from that of host, vacancies are created in the crystal lattice of the host.
(iii) For example, addition of CdCl2 to silver chloride yields solid solutions where the divalent cation Cd2+ occupies the position of Ag+.
(iv) This will disturb the electrical neutrality of the crystal.
(v) In order to maintain the same, proportional number of Ag+ ions leaves the lattice.
(vi) This produces a cation vacancy in the lattice, such kind of crystal defects are called impurity defects.
Given data: Time taken for completion of 30% of the reaction = 12 mins.
a = 100; x = 30; a - x = 70 and t =12 min
Formula: \(k=\frac { 2.303 }{ t } \log { \frac { a }{ a-x } } \)
Solution:
For a first order reaction,
\(\frac { 2.303 }{ 12 } \log\frac { 100 }{ 70 } \)
\(=\frac { 2.303 }{ 12 } \times 0.1549\) = 0.02972 min-1
k = 2.97 x 10-2 min-1
If t = 65.33 minutes, x = ?
\(k=\frac { 2.303 }{ 65.33 } \log\frac { 100 }{ 100-x } \)
\(0.02972=\frac { 2.303 }{ 65.33 } \log\frac { 100 }{ 100-x } \)
\(\log\frac { 100 }{ 100-x } \)= \(\frac { 0.02972\times 65.33 }{ 2.303 } =0.8430\)
\(\frac { 100 }{ 100-x }\) =Antilog of 0.8430
100 = 6.966 (100 - x)
100 = 696.6 - 6.966x
-6.966x = 100-696.6 = 596.6
\(x=\frac { 596.6 }{ 6.966 } =85.62%\)
The reaction completed in 65.33 minutes
pH = - log [H3O+]
(i) pH - log [104]
pH = log 1 - log 104
pH = - 4
(ii) pH = - log [10-7]
pH = log 1 - log 10-7.
pH = 7
(iii) pH = - log [H3O+]
pH = - log [6.8 x 10-3]
pH = log 1 - log 6.8 - log 10-3
= 3 - 0.8325
pH = 2.17 (or) 2.2
(iv) pH = - log [3.2 x 10-5]
pH = log 1 - log 3.2 - log 10-5
= 5 - 0.5051
pH = 4.49 (or) 4.5
(v) pH = - log [0.035]
pH = log 1 - log 0.035
= 2 - 0.5441
pH = 1.46 (or) 1.5
(vi) pH = - log [0.25]
pH = log 1 - log 0.25
= 1 - 0.3979
pH = 0.602 (or) 0.60
(vii) pH = -log [H3O+]
pH = log 1 - log 5.4 - log 10-9
= 7 - 0.7324
pH = 8.267 (or) 8.3
(viii) pH = - log [7.1 x 10-7]
pH = log 1 - log 7.1 - log 10-7
= 7 - 0.8513
pH = 6.2.
Sn4+ + 2e- ⟶ Sn2+ E0 = 0.15V
Given: n = 2 electrons
F = 96495 coulombs
Formula: ΔG = - nFEO
Solutlon: ∴ ΔG = - 2 x 96495 x 0.15
= 28.948
Free energy = -28.948 kJ.











