CBSE 10th Standard Science Subject Carbon and Its Compounds HOT Questions 2 Mark Questions With Solution 2021
By QB365 on 29 May, 2021
QB365 Provides the HOT Question Papers for Class 10 Science, and also provide the detail solution for each and every HOT Questions. HOT Questions will help to get more idea about question pattern in every exams and also will help to get more marks in Exams
QB365 - Question Bank Software
CBSE 10th Standard Science Subject Carbon and Its Compounds HOT Questions 2 Mark Questions With Solution 2021
10th Standard CBSE
-
Reg.No. :
Science
-
A, B, C are members of homologous series and their melting points are -183°C, -138°C, 130°C respectively. Among these
(i) Which member will have least number of carbon atoms?
(ii) Which member will have maximum number of carbon atoms?(a) -
A hydrocarbon compound A is active ingredient of wine and cough syrup. A on oxidation with acidified K2Cr2O7 forms compound B. Identify the compound A and B and write the chemical equations involved.
(a) -
Write an activity to show the acidic nature of ethanol. Give the chemical equation of the reaction taking place
(a) -
A compound 'X' has molecular formula C2H60 is saturated hydrocarbons and is a very good solvent. How can you convert it into unsaturated hydrocarbon? Identify X and show its conversion with the help of equation.
(a) -
Take about 20 mL of castor oil in a beaker. Add 30 mL of 20% sodium hydroxide solution. Heat the mixture with continuous stirring for a few minutes till the mixture thickens. Add 5-10 g of common salt to this. Stir the mixture well, allow it to cool, soaps is obtained.
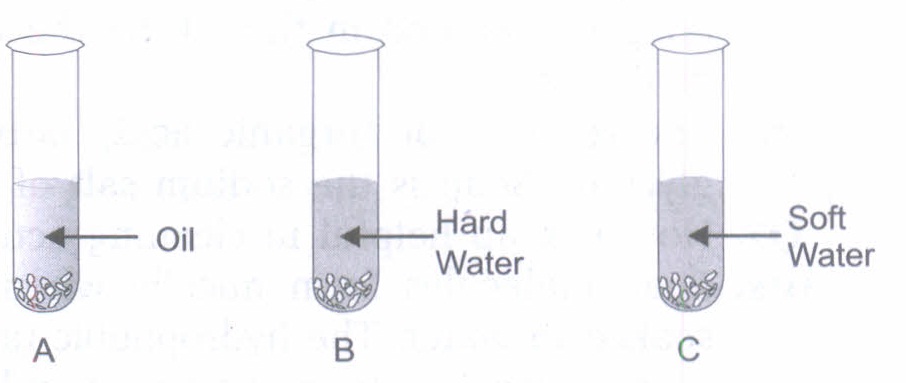
(a) Why do we use common salt to make soap?
(b) What will happen if you will add the above made soap solutions to the following test tubes A, B, and C.
(c) Can we use potassium hydroxide instead of sodium hydroxide.(a)
*****************************************
CBSE 10th Standard Science Subject Carbon and Its Compounds HOT Questions 2 Mark Questions With Solution 2021 Answer Keys
-
(i) A will have least number of carbon atoms.
(ii) C will have maximum number of carbon atoms. -
A is ethanol, C2H5OH
B is ethanoic acid, CH3COOH
Equation: C2H5OH \(\overset {acidified \ K_2Cr_2O_7}{\longrightarrow}\) CH3COOH -
Take ethanol in a test tube and drop a small piece of sodium about the size of a grain of rice into it. The reaction evolves a colourless gas which is hydrogen.
Hydrogen gas can be tested by bringing a burning splinter/matchstick near the mouth of the test tube, it bums with the popping sound.
This activity proves that ethanol like other acids release H2 gas
2Na + 2CH3CH2OH \(\longrightarrow\) 2CH3CH2ONa + H2 -
'X' is CH3-CH2OH ethanol. It can be made unsaturated by heating it with cone. H2SO4 which is a dehydrating agent and removes water from it, thereby forming ethene.
CH3-CH2OH \(\xrightarrow[{}{H_2SO_4}]{Hot \ Conc.}\) H2C = CH2 + H2O
Ethene -
(a) Salt: NaCI is added while making soap, because it will cause precipitation of soap in solid form.
(b) In test tube A: Soap + Oil \(\longrightarrow\) Lather/foam is formed. Carbon chain of carboxylic salt dissolves in oil.
In test tube B: Soap + Hard water \(\longrightarrow\) Insoluble compound called scum is formed.
In test tube C: Soap + soft water \(\longrightarrow\) Froth is formed.
(c) Yes, we can use potassium hydroxide to prepare soap.

























