CBSE 10th Standard Science Subject Periodic Classification of Elements Chapter Case Study Questions 2021
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 10 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 10th Standard Science Subject Periodic Classification of Elements Case Study Questions 2021
10th Standard CBSE
-
Reg.No. :
Science
-
Generally metals possessing 1, 2 or 3 electrons in their respective valence shells have a strong tendency to lose electrons to form positive ions. Non-metals, on the other hand, having 4 to 8 electrons in their respective outermost shells generally have a tendency to gain electrons to form negative ions. Metallic character is called electropositive character and non-metallic character is called electronegative character. The metallic character increases down a group and non-metallic character increases along a period.
(i) Which of the following electronic configurations represent most electropositive element?(a) 2,1 b) 2,8,1 (c) 2,2 (d) 2,8,2 (ii) Considering the elements B, C, N, F and Si, the correct order of their non-metallic character is
(a) B>C>Si>N>F b) Si>C>B>N>F c) F>N>C>B>Si (d) F>N>C>Si>B (iii) Which of the following is least metallic?
(a) N (b) P (c) As (d) Sb (iv) To which of the following categories does the element with atomic number 14 belong?
(a) Metal (b) Metalloid (c) Non-metal (d) Left-hand side element (v) Non-metals are present in the periodic table at
(a) right side (b) left side (c) middle (d) both right and left (a) -
The distance between the centre of the nucleus and the outermost shell of electrons is known as atomic radius.On moving from left to right along a period, atomic radii decrease because effective nuclear charge increases.
For example, the atomic size decreases regularly from Li to F in the second period and from Na to CI in the third period. It may, however, be noted that in any period, the noble gas has the largest size. On moving down in a group, atomic radii increase.
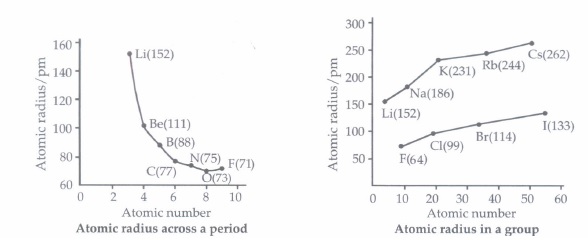
(i) Which of the following has the maximum atomic radius?(a) Al (b) Si (c) P (d) Mg (ii) The element with the smallest size in group l3 is
(a) gallium (b) thallium (c) aluminium (d) boron (iii) The atomic radius decreases as we move across a period because
(a) atomic mass increases
(b) atomic number decreases
(c) effective nuclear charge increases
(d) electrons are removed
(iv) In the third period of the periodic table, the element having smallest size is(a) Na (b) Ar (c) CI (d) Si (v) Among O, C, F, CI, Br, the correct order of increasing atomic radii is
(a) F, O, C, CI, Br (b) F, C, O, CI, Br (c) F, Cl, Br, O, C (d) C, O, F, Cl, Br (a) -
Study the following table in which positions of six elements A, B, C, D, E and F are shown as they are in the modern periodic table:
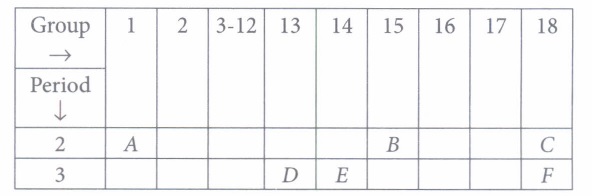
(i) Which element in the given table has same number of electrons as in K+ and CI-.(a) C (b) F (c) E (d) D (ii) The formula of the oxide of element D will be
(a) DO (b) D2O (c) D2O3 (d) D2O5 (iii) Which of the following elements has most metallic character?
(a) F (b) D (c) E (d) B (iv) Element E forms a chloride with formula
(a) ECl2 (b) ECl3 (c) ECl4 (d) ECI (v) Which of the following elements is a metalloid?
(a) A (b) B (c) C (d) E (a) -
The recurrence of properties of the elements after a certain regular intervals, when they are arranged in the increasing order of their atomic numbers, is called periodicity. There are a number of physical properties such as atomic size, metallic and non -metallic character, etc. which show periodic variation. In periodic table, various properties vary differently from moving left to right in a period and going down in a group. In a period, properties vary because from moving left to right in a period, number of shells remain same but valence electron increases by one number hence nuclear charge increases. In a group, on going down, number of valence shells increases while number of valence electrons remains same.
(i) From top to bottom in a group of the periodic table, the electropositive character of the element(a) increases (b) decreases (c) remains unchanged (d) changes irregularly (ii) Which element has the largest size in the second period?
(a) N (b) F (c) Li (d) Be (iii) Which of the following elements has three valence electrons?
(a) Cs (b) Ca (c) Al (d) S (iv) In the periodic table, the metallic character of elements
(a) decreases from left to right and decreases down the group
(b) decreases from left to right and increases down the group
(c) increases from left to right and increases down the group
(d) increases from left to right and decreases down the group
(v) Which of the following increases along the period?(a) Number of valence electrons (b) Atomic size (c) Electropositive character (d) All of these (a) -
"Properties of elements are the periodic function of their atomic numbers".This is known as modern periodic law. It means that the properties of elements depend on their atomic numbers and the elements are given positions in the periodic table on the basis of their increasing atomic number. Atomic number determines the distribution of electrons in the orbit, and electrons of the outermost orbit determine the properties of an element. There are 18 groups (vertical columns) and 7 periods (horizontal lines) in modern form of the periodic table. The number of the period is equal to the number of shells in the atoms of the elements belonging to that period.
(i) What is the atomic number of element of period 3 and group 17?(a) 10 (b) 14 (c) 17 (d) 12 (ii) Atomic number of an element is 2, 8, 6. Its period number and valency are respectively
(a) 3,2 (b) 6,6 (c) 6,2 (d) 2,2 (iii) An element has mass number 40 and contains 20 neutrons in its atom. To which period and group of the periodic table does it belong?
(a) Period-3, Group-3 (b) Period-4, Group-3 (c) Period-3, Group-2 (d) Period-4, Group-4 (iv) An elements 'X' has an atomic number of 16. With which of the following elements will it show similar chemical properties?
(a) Ne (10) (b) N (7) (c) O (8) (d) Be (4) (v) Identify the statement(s) which is(are) true for the modern periodic table.
(a) It reflects trends in physical and chemical properties of the elements.
(b) It helps to reflect the relative atomicity of bonds between any two elements.
(c) It helps to predict the stable valency state of the elements.
(d) All of these(a)
*****************************************
CBSE 10th Standard Science Subject Periodic Classification of Elements Case Study Questions 2021 Answer Keys
-
(i) (b): Metallic character or electropositive character increases down a group and decreases
along a period. The given elements are Li, Na, Be and Mg respectively. Among these elements, Na is most electropositive.
(ii) (c): Non-metallic character increases left to right in a period and decreases from top to bottom in a group.
(iii) (a)
(iv) (b): The element with atomic number 14 is Si which is a metalloid.
(v) (a) -
(i) (d): In general, the atomic radii decrease along a period and increase down a group.
\(\begin{array}{ccccc} \text { Atom } & { }_{12} \mathrm{Mg} & { }_{13} \mathrm{Al} & { }_{14} \mathrm{Si} & { }_{15} \mathrm{P} \\ \text { Radius }(\mathrm{pm}) & 160 & 143 & 118 & 110 \end{array}\)
Thus, Mg has maximum atomic radius.
(ii) (d): Boron is the first element of group 13, hence it is smallest in size
(iii) (c) : Effective nuclear charge increases along a period and due to addition of electrons in the same shell it causes the incoming electron to experience more force of attraction by the nucleus.
Therefore, the size of the atom decreases.
(iv) (c): Atomic size decreases across the period. CI has smaller size than Ar. Argon has larger atomic size as compared to CI due to the inert nature (it has completely filled outer shell).
(v) (a): Atomic size decreases from left to right in a period and increases from top to bottom in a group.
Thus, the order is F < O< C < CI < Br. -
(i) (b): F is argon which has atomic number 18. It has 18 electrons. K+and CL- ions also have 18 electrons each.
(ii) (c): D is aluminium which is an element of group 13. Valency of aluminium is 3. Hence, the formula of its oxide will be Al2O3
(iii) (b): D is aluminium, which has the most metallic character among the given elements.
(iv) (c): Valency of E is 4. Hence, the formula of the chloride will be ECI4.
(v) (d): E is silicon which is a metalloid. -
(i) (a): As the size of the atom increases down the group, electropositive character increases.
(ii) (c): Li is the first element of the second period. As the size decreases in the period from left to right, therefore, Li is the largest atom in the period.
(iii) (c): Al (Z = 13) : 2, 8, 3
(iv) (b): Metallic character of elements decreases from left to right and increases down the group.
(v) (a): As we move from left to right along a period, the number of valence electrons increases from 1 to 8. -
(i) (c) :The element is chlorine (Z = 17).
(ii) (a): The element (sulphur) belongs to third period and its valency is 2.
period and its valency is 2.
(iii) (c) : Atomic number of the element = 40 - 20 = 20.Electronic configuration of the element is 2, 8, 8, 2; i.e.,the element is calcium which belongs to 4th period and 2nd group of the periodic table.
(iv) (c): The element is sulphur. Sulphur and oxygen belong to group 16.
(v) (d)

























