CBSE 11th Standard Chemistry Subject HOT Questions 2 Mark Questions With Solution 2021
By QB365 on 28 May, 2021
QB365 Provides the HOT Question Papers for Class 11 Chemistry, and also provide the detail solution for each and every HOT Questions. HOT Questions will help to get more idea about question pattern in every exams and also will help to get more marks in Exams
QB365 - Question Bank Software
CBSE 11th Standard Chemistry Subject HOT Questions 2 Mark Questions With Solution 2021
11th Standard CBSE
-
Reg.No. :
Chemistry
-
In Rutherford’s experiment, generally the thin foil of heavy atoms, like gold, platinum etc. have been used to be bombarded by the α-particles. If the thin foil of light atoms like aluminium etc. is used, what difference would be observed from the above results ?
(a) -
Aluminium forms the ion Al3+ , but not Al 4+ why?
(a) -
Discuss the hybridisation of Be in gaseous state and solid state.
(a) -
Use the information and data given below to answer the question, stronger intermolecular forces result in higher boiling point.
Strength of London forces increases with the number of electrons in the molecules. Boiling point of HF, HCI, HBr, and HI are 293 K, 189 K, 206 K and 238 K respectively.
Looking at the trend of boiling points of HCI, HBr, and HI, explain out of dipole-dipole interaction and London interaction, which one is predominant here.(a) -
Use the information and data given below to answer the question, stronger intermolecular forces result in higher boiling point.
Strength of London forces increases with the number of electrons in the molecules. The boiling point of HF, HCI, HBr, and HI are 293 K, 189 K, 206 K and 238 K respectively.
Why is the boiling point of hydrogen fluoride highest while that of hydrogen chloride lowest?(a) -
Why standard entropy of an elementary substance is not zero whereas standard enthalpy of formation is taken as zero?
(a) -
Calculate the free energy change when 1 mole of NaCl is dissolved in water at 298 K. (Given, lattice energy of NACl = -777.8 kJ mol-1 , hydration energy = -774.1 kJ mol-1 and \(\Delta S=0.043kJ \ K^{ -1 }mol^{ -1 }\) at 298 K).
(a) -
Give reasons.
A mixture of dilute NaOH and aluminium pieces is used to open drain.(a) -
A compound A of boron reacts with NMe3 to give an adduct B which on hydrolysis gives a compound C and hydrogen gas. Compound C is an acid. Identify the compounds A, B and C. Give the reactions involved.
(a) -
Carbon monoxide is readily absorbed by ammonical cuprous chloride solution but carbon dioxide is not. Explain.
(a)
*****************************************
CBSE 11th Standard Chemistry Subject HOT Questions 2 Mark Questions With Solution 2021 Answer Keys
-
Heavy atoms such as gold, platinum have nucleus. Heavy nucleus contains large amount of positive charge. When a beam of \(\alpha \)-particles is shot at a thin gold foil, most of them pass through without much effect.
Some however, are deflected back or by small angles due to enormous repulsive force of heavy nucleus. If light aluminium foil is used, the number of \(\alpha \) -particles deflected back or those deflected by small angles will be negligible. -
Aluminium \(([Ne]^{ 3{ s }^{ 2 } } \ 3p'\) can achien the electronic configuration of the nearest noble gas (Ne) by losing only three electrons, Al3+ = 1s22s22p6 . Aluminium with not form the Al4+ ion because an extremely high amount of energy would be required to remove an electron from the stable noble gas configuration.
-
In gaseous state at high temperature, BeCl2 exists as linear molecule Cl-Be-Cl, thus the hybridisation of the central atoms is sp

In solid state,it has a polymeric structure with chlorine bridges as follows
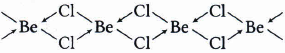
Two Cl-atoms are listed to be atom by two coordination bonus and two by covalent bonds. For these bonds to be formed, Be in the excited state with the configuration undergoes Sp3 hybridization. Two half-filled hybrid orbitals will form normal covalent bonds with two Cl-atoms. The other two Cl-atoms are coordinated to Be-atom. The other two Cl-atoms are coordinated be Be-atom by donating electron pairs into the empty hybrid orbitals. -
From the information and data given in the question, we concluded that Electronegativity of chlorine, bromine, and iodine decreases in the following order.
CI > Br >
Therefore, dipole should moment decrease from HCI to HI. As a result, dipole-dipole interaction should also decrease from HCI to HI. But boiling point increases on moving from HCI and HI. This means that London forces are predominant.
This is so because London forces increases as the number of electrons in a molecules increases and in this case, number of electrons is increasing from HCI towards HI. -
From the information and data given in the question, we concluded that Hydrogen fluoride has highest dipole moment is due to highest electronegativity of fluorine as well as due to the presence of hydrogen bonding in HF. Therefore, HF has highest boiling point.
-
A substance has a perfectly ordered arrangement of its constituent particles only at absolute zero. Hence, entropy is zero only at absolute zero. Enthalpy of formation is the heat change involved in the formation of one mole of the substance from its elements. An element formed from itself means no heat change, i.e \(\Delta _{ f }H^{ \circ }=0\) .
-
\(\Delta H=hydration \ energy-lattice \ energy\)
\(\Delta H=-774.1kJmol^{ -1 }-(-777.8kJmol^{ -1 })=3.7kJmol^{ -1 }\)
\(\Delta G=-9.11kJmol^{ -1 }\) -
NaOH reacts with Al to evolve dihydrogen gas. The pressure of the hydrogen gas can be used to open drains.
2Al(s) + 2NaOH(aq) + 2H2O(l) \(\rightarrow \) 2NaAlO2(aq) + 2H2(g) -
Since, compound a of boron reacts with NMe3 to form an adduct B. Thus, compound A is a Lewis acid. Since adduct B on hydrolysis gives an acid C and hydrogen gas, therefore, A is B2H6 and C is boric acid.
\(B_{ 2 }{ H }_{ 6 }+2NMe_{ 3 }\rightarrow 2BH_{ 3 }.NMe_{ 3 }\)
Diborate(A) Adduct(B)
\(BH3.NMe_{ 3 }+3H_{ 2 }O\rightarrow H_{ 3 }BO_{ 3 }+NMe_{ 3 }+6{ H }_{ 2 }\)
Boric acid(C) -
Due to the presence of a lone pair of electrons on carbon in CO, it acts as as a Lewis base (or ligand) and thus forms a soluble complex with ammoniacal cuprous chloride solution.
CuCl + NH3+ :CO\(\longrightarrow \)[Cu(CO)NH3]+Cl-
Soluble complex
On the other hand, CO2 does not act as a Lewis Base since it does not have a lone pair of electrons on the carbon and hence, does not dissolve in ammoniacal cuprous chloride solution.



































