CBSE 11th Standard Chemistry Subject HOT Questions 3 Mark Questions 2021
By QB365 on 28 May, 2021
QB365 Provides the HOT Question Papers for Class 11 Chemistry, and also provide the detail solution for each and every HOT Questions. HOT Questions will help to get more idea about question pattern in every exams and also will help to get more marks in Exams
QB365 - Question Bank Software
CBSE 11th Standard Chemistry Subject HOT Questions 3 Mark Questions 2021
11th Standard CBSE
-
Reg.No. :
Chemistry
-
Which of the following sets of orbitals are degenerate and why?
(a) 1s, 2s and 3s in Mg - atom.
(b) 2px, 2py and 2pz in C-atom.
(c) 3s, 3px and 3d - orbitals in H - atom.(a) -
Iron piece are attracted toward a magnet but zinc piece are not Why?
(a) -
Indicate the type of bonds present in NH4No3 and state the mode of hybridisation of two N-atoms in it.
(a) -
Density of a gas is found to be 5.46 g/dm3 at 27 C and 2 bar pressure. What will be its density at STP?
Notes: STP mean at 1 bar and 273 K, so compare the density at 27\(^{o}\) C and 2 bar with density at STP by using the relation d = pM/RT(a) -
Prove that the excluded volume 'b' is four times the actual volume of the gas molecules.
(a) -
50.0 g of CaCO3 are heated to 1073 K in a 5L vessel. What percent of the CaCO3 would decompose at equilibrium? Kp for the reaction.
\({ CaCO }_{ 3 }(s)\rightleftharpoons CaO(s)+{ CO }_{ 2 }(g)\) is 1.15 a/m at 1073 K.(a) -
Describe the industrial applications of hydrogen dependent on its effect on the unsaturated organic systems in the presence of a catalyst.
(a) -
A tetravalent element forms monoxide and dioxide withoxygen. When air is passed over heated element(1273 K), Producer gas is obtained. Monoxide of the element is powerful reducing agent and reduces ferric oxide to iron. Identify the element and write formulas of its monoxide and dioxide.
Write chemical equations for the formation of prodecur gas and reduction of ferric oxide with the monoxide.(a) -
An alkane has a molecule mass of 72. Give all the possible structural isomers along with their IUPAC names.
(a)
*****************************************
CBSE 11th Standard Chemistry Subject HOT Questions 3 Mark Questions 2021 Answer Keys
-
(a) 1s, 2s and 3s - orbitals in Mg-atom are not degenerate because these have different values of n.
(b) 2px, 2py and 2pz - orbitals in C-atom are degenerate because these belong to same subshell.
(c) 3s, 3px and 3d - orbitals in H - atom are degenerate because for H-atom, the subshells having same value of n have same energies. -
Electronic configuration of iron suggests that it contains unpaired electrons\({ 26 }^{ fe }=[Ar{ ] }^{ 18 }{ 3d }^{ 6 }{ 4s }^{ 2 }\)
\(\upharpoonleft \downharpoonright \) \(\upharpoonleft \) \(\upharpoonleft \) \(\upharpoonleft \) \(\upharpoonleft \) \(\upharpoonleft \downharpoonright \) and hence it is perpendicular whereas has no unpaired electron
\({ 26 }^{ fe }=[Ar{ ] }^{ 18 }{ 3d }^{ 6 }{ 4s }^{ 2 }\)and hence, it is diamagnetic. it is rather repelled by the magnet. -
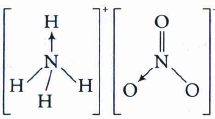
\({ NH }_{ 4 }^{ + }\) ion contains covalent and dative bonds. (It is formed by donation of lone pair of electrons on N in NH3 to H+ ion). \({ NO }_{ 3 }^{ - }\) ion also contains covalent and davite bonds.
Bond between \({ NH }_{ 4 }^{ + }\) and \({ NO }_{ 3 }^{ - }\) ions is ionis.
N of \({ NH }_{ 4 }^{ + }\) ion is sp3 hydridised and is tetrahedral.
N of \({ NO }_{ 3 }^{ - }\) ion is sp2 hybridised and is planar. -
Density, d = pM/RT
For same gas at different temperatures and pressures
\(\cfrac { { d }_{ 2 } }{ d_{ 1 } } =\cfrac { { p }_{ 2 }{ T }_{ 1 } }{ { p }_{ 1 }{ T }_{ 2 } }\)
\( { d }_{ 2 }=\cfrac { { p }_{ 2 }{ T }_{ 1 }d_{ 1 } }{ { p }_{ 1 }{ T }_{ 2 } } \)
\(\cfrac { 1\times 300\times 5.46 }{ 2\times 273 } \)
= 3 g dm-3 -
Consider two molecules to be spherical, if r is the radius of the molecules then the distance of closest approach between the two molecules = 2r (as shown in Figure). This is the distance between the centres of their nuclei.
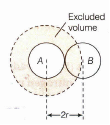
Since the molecules cannot come closer than distance 2r, the excluded volume for a pair of molecules = volume of sphere of radius 2r
\(=\frac { 4 }{ 3 } \pi { \left( 2r \right) }^{ 3 }=8\times \frac { 4 }{ 3 } \pi { r }^{ 3 }\)
\(\therefore \) Excluded volume per molecules (b)
\(=\frac { 1 }{ 2 } \left( 8\times \frac { 4 }{ 3 } \pi { r }^{ 3 } \right) =4\times \frac { 4 }{ 3 } \pi { r }^{ 3 }\)
But \(\frac { 4 }{ 3 } \pi { r }^{ 3 }={ V }_{ m }\), i.e. the actual volume of the gas molecule.
\(\therefore \) b = 4Vm -
\({ CaCO }_{ 3 }(s)\rightleftharpoons CaO(s)+{ CO }_{ 2 }(g)\)
Kp = \({ p }_{ { CO }_{ 2 } }\) = 1.15 atm, pV = nRT
\({ N }_{ { CO }_{ 2 } }=\frac { { P }_{ { CO }_{ 2 } } }{ RT } =\frac { 1.15\times 5 }{ 0.082\times 1073 } =0.065mol\)
1 mole of CO2 is obtained by decomposition of 1 mole CaCO3. Therefore, moles of CaCO3 decomposed is equal to the moles of CO2 = 0.065 mol.
Moles of CaCO3 initially present = \(\frac { 50 }{ 100 } =0.5 \ mol\)
[Molecular mass of CaCO3 = 100]
Percent of CaCO3 decomposed \(=\frac { 0.065 }{ 0.5 } \times 100=13\)% -
Due to this property hydrogen is used for the manufacture of vanaspati ghee from edible oils such as cotton-seed oil, soyabean oil, corn oil etc.
\(unsaturated \ oil \ + \ H_{ 2 }(g)\overset { Ni \ catalyst }{ \longrightarrow } Vanaspati\quad ghee\) -
Producer gas is a mixture of CO and N2, therefore, the tetravalent element is carbon and its monoxide and dioxide are CO and CO2 respectively.
\(2C(s)+\underbrace { O_{ 2 }(s)+4N_{ 2 }(g) }_{ Air } \overset { 1273k }{ \rightarrow } \underbrace { 2CO(g)+4N_{ 2 }(g) }_{ Producer \ gas } \)
The carbon monoxide is a strong reducing agent and reduces ferric oxide to iron.
\(Fe_{ 2 }O_{ 3 }(s)+3CO(g)\overset { \Delta }{ \rightarrow } 2Fe(s)+3CO_{ 2 }(g)\) -
The general formula of alkanes is CnH2n+2=12xn+1x(2n+2)=72 or 12n+2n+2=72 or n=5
Thus, the molecular formula of the alkane is C5H12. For structural isomers and their IUPAC names.



































