CBSE 11th Standard Chemistry Subject HOT Questions 3 Mark Questions With Solution 2021
By QB365 on 28 May, 2021
QB365 Provides the HOT Question Papers for Class 11 Chemistry, and also provide the detail solution for each and every HOT Questions. HOT Questions will help to get more idea about question pattern in every exams and also will help to get more marks in Exams
QB365 - Question Bank Software
CBSE 11th Standard Chemistry Subject HOT Questions 3 Mark Questions With Solution 2021
11th Standard CBSE
-
Reg.No. :
Chemistry
-
Prove that density of the nucleus is constant.
(a) -
What is meant by the term bond order? Calculate the bond order of : N2, O2, O2 +, \({ O }_{ 2 }^{ - }\)
(a) -
Indicate the type of bonds present in NH4No3 and state the mode of hybridisation of two N-atoms in it.
(a) -
A man takes a diet equivalent to 10000 kJ per day and does work, in expending his energy in all forms equivalent to 12500 kJ per day. What is change in internal energy per day?If the energy lost was stored as sucrose (1632 kJ pre 100g), how many days should it take to lose 2kg of his weight?(Ignore water loss)
(a) -
What is the minimum volume of water required to dissolve 1g of calcium sulphate at 298 K? (For calcium sulphate, Ksp is 9.1 x 10-6).
(a) -
Describe the industrial applications of hydrogen dependent on the heat liberated when its atoms are made to combine on the surface of a metal.
(a) -
Due to this property hydrogen is used for the manufacture of vanaspati ghee from edible oils such as cotton-seed oil, soyabean oil, corn oil etc.
(a) -
A tetravalent element forms monoxide and dioxide withoxygen. When air is passed over heated element(1273 K), Producer gas is obtained. Monoxide of the element is powerful reducing agent and reduces ferric oxide to iron. Identify the element and write formulas of its monoxide and dioxide.
Write chemical equations for the formation of prodecur gas and reduction of ferric oxide with the monoxide.(a) -
An alkane has a molecule mass of 72. Give all the possible structural isomers along with their IUPAC names.
(a)
*****************************************
CBSE 11th Standard Chemistry Subject HOT Questions 3 Mark Questions With Solution 2021 Answer Keys
-
Radius of the nucleus = \(1.33\times { 10 }^{ -11 }\times A^{ 1/3 }m\)
Density of nucleus \(=\frac { mass }{ volume } \)
\(=\frac { A\times 1.66\times 10^{ -27 }kg }{ \frac { 4 }{ 3 } \times \pi \times (1.33\times 10^{ -11 }\times { A }^{ 1/3 })^{ 3 } } \)
= constant
Thus, density of nucleus is constant, independent of the element under consideration. -
Bond order is defined as one half the difference between the number of electron present in the bonding and anti-bonding orbitals, i.e
Bond order (BO) \(=\frac { 1 }{ 2 } ({ N }_{ b }-{ N }_{ a })\)
A positive bond order means a stable molecule while a negative or zero bond order means an unstable molecule.
Stability of a molecule \(\propto \) bond order
Bond length \(\propto \) \(\frac { 1 }{ bond \ order } \)
Bond order values 1,2 or 3 corresponding to single, double or triple bonds respectively. -
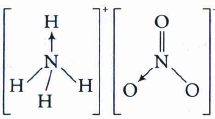
\({ NH }_{ 4 }^{ + }\) ion contains covalent and dative bonds. (It is formed by donation of lone pair of electrons on N in NH3 to H+ ion). \({ NO }_{ 3 }^{ - }\) ion also contains covalent and davite bonds.
Bond between \({ NH }_{ 4 }^{ + }\) and \({ NO }_{ 3 }^{ - }\) ions is ionis.
N of \({ NH }_{ 4 }^{ + }\) ion is sp3 hydridised and is tetrahedral.
N of \({ NO }_{ 3 }^{ - }\) ion is sp2 hybridised and is planar. -
Energy taken by a man = 10000 kJ
Change in internal energy per day = 12500 - 10000 = 2500 kJ
The energy is lost by the man as he expends more energy than he takes.Now 100g of sugar corresponds to energy = 1632 kJ loss in energy.
2000g of sugar corresponds to energy = \(\frac { 1632\times 2000 }{ 100 } \)
= 32640 kJ
Number of days required to lose 2000g of weight or 32640 kJ of energy = \(\frac { 32640 }{ 2500 } \) = 13days -
\({ CaSO }_{ 4 }\rightleftharpoons { Ca }^{ 2+ }+{ SO }_{ 4 }^{ 2- };{ K }_{ sp }=9.1\times { 10 }^{ -6 }\)
S S S
Where s is the solubility of CaSO4
\({ K }_{ sp }=\left[ { Ca }^{ 2+ } \right] \left[ { SO }_{ 4 }^{ 2- } \right] =S.S={ S }^{ 2 }\)
\(S=\sqrt { { K }_{ sp } } =\sqrt { 9.1\times { 10 }^{ -6 } } \Rightarrow S=3.017\times { 10 }^{ -3 }M\)
Solubility of CaSO4 = 3.017 x 10-3 mol-1
= 3.017 x 10-3 x 136 gL-1
(Molar mass of CaSO4 =136 g mol-1)
= 410.3 x 10-3 gL-1
410.3x10-3 g CaSO4 is dissolved in = 1L
1g CaSO4 is dissolved in = \(\frac { 1\times 1 }{ 410.3\times { 10 }^{ -3 } } \) = 2.437 L -
Due to this property hydrogen is used in atomic hydrogen welding/cutting torch.
-
Due to this property dihydrogen is used for the manufacture of ammonia (Haber's process)
\(N_{ 2 }(g)+3H_{ 2 }(g)\overset { 673K, \ 200 \ atm }{ \underset { Fe,Mo }{ \rightleftharpoons } } 2NH_{ 3 }(g)\) -
Producer gas is a mixture of CO and N2, therefore, the tetravalent element is carbon and its monoxide and dioxide are CO and CO2 respectively.
\(2C(s)+\underbrace { O_{ 2 }(s)+4N_{ 2 }(g) }_{ Air } \overset { 1273k }{ \rightarrow } \underbrace { 2CO(g)+4N_{ 2 }(g) }_{ Producer \ gas } \)
The carbon monoxide is a strong reducing agent and reduces ferric oxide to iron.
\(Fe_{ 2 }O_{ 3 }(s)+3CO(g)\overset { \Delta }{ \rightarrow } 2Fe(s)+3CO_{ 2 }(g)\) -
The general formula of alkanes is CnH2n+2=12xn+1x(2n+2)=72 or 12n+2n+2=72 or n=5
Thus, the molecular formula of the alkane is C5H12. For structural isomers and their IUPAC names.



































