CBSE 11th Standard Chemistry Subject Organic Chemistry: Some Basic Principles and Techniques Ncert Exemplar 2 Mark Questions With Solution 2021
By QB365 on 26 May, 2021
QB365 Provides the updated NCERT Exemplar Questions for Class
11, and also provide the detail solution for each and every NCERT
Exemplar questions. NCERT Exemplar questions are latest updated
question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 11th Standard Chemistry Subject Organic Chemistry: Some Basic Principles and Techniques Ncert Exemplar 2 Mark Questions With Solution 2021
11th Standard CBSE
-
Reg.No. :
Chemistry
-
Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens?
(a) -
Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
(a) C6H5OH
(b) C6H5NO2
(c) CH3CH = CHCHO
(d) C6H5-CHO
(e) C6H5-CH2
(f) CH3CH = CHCH2(a) -
Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.CH3CH = CHCHO
(a) -
Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.C6H5-CHO
(a) -
Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.C6H5-CH2
(a)
*****************************************
CBSE 11th Standard Chemistry Subject Organic Chemistry: Some Basic Principles and Techniques Ncert Exemplar 2 Mark Questions With Solution 2021 Answer Keys
-
sodium extract is boiled with nitric acid to decompose NaCN and Na2S if present.
\(\mathrm{NaCN}+\mathrm{HNO}_{3} \longrightarrow \mathrm{NaNO}_{3}+\mathrm{HCN} \uparrow\)
\(\mathrm{Na}_{2} \mathrm{~S}+2 \mathrm{HNO}_{3} \longrightarrow 2 \mathrm{NaNO}_{3}+\mathrm{H}_{2} \mathrm{~S} \uparrow\)
If cyanide and sulphide are not removed, they will react with AgNO3 and hence, will interfere with the silver nitrate test for halogens
\(\mathrm{NaCN}+\mathrm{AgNO}_{3} \longrightarrow \underset{\text { White ppt }}{\mathrm{AgCN}}+\mathrm{NaNO}_{3}\)
\(\mathrm{Na}_{2} \mathrm{~S}+2 \mathrm{AgNO}_{3} \longrightarrow \underset{\text { Black } \mathrm{ppt}}{\mathrm{Ag}_{2} \mathrm{~S}}+2 \mathrm{NaNO}_{3}\) -
(a)
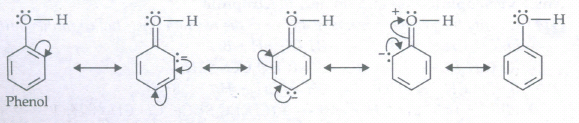
(b)
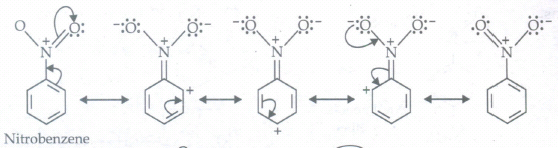
(c)
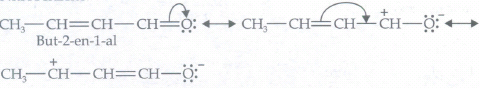
(d)
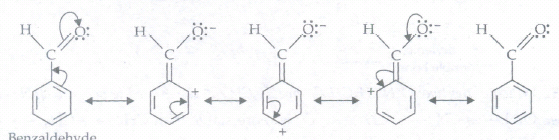
(e)
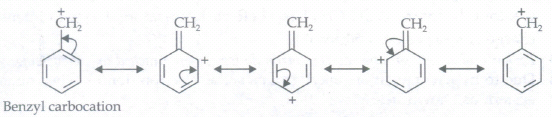
(f)
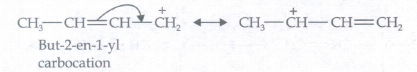
-

-

-




































