CBSE 12th Standard Chemistry Subject Aldehydes , Ketones and Carboxylic Acids Ncert Exemplar 2 Mark Questions 2021
By QB365 on 25 May, 2021
QB365 Provides the updated NCERT Exemplar Questions for Class 12, and also provide the detail solution for each and every NCERT Exemplar questions. NCERT Exemplar questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 12th Standard Chemistry Subject Aldehydes , Ketones and Carboxylic Acids Ncert Exemplar 2 Mark Questions 2021
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Pentan-2-one and Pentan-3-one
(ii) Ethanal and Propanal(a) -
Benzaldehyde can be obtained from benzal chloride. Write reactions obtaining benzalchloride and then benzaldehyde from it.
(a) -
Compound 'A' was prepared by oxidation 'B' with alkaline KMnO4. Compound 'A' on reduction with lithium aluminium hydride gets converted back to compound 'B'. When compound 'A' is heated with compound B in the presence of H 2SO4 it produces fruitly smell of compound C to which family the compounds 'A', 'B' and 'C' belong to?
(a) -
Alkenes,
 and carbonyl compounds ,
and carbonyl compounds ,  both contain a \(\pi\) - bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.(a)
both contain a \(\pi\) - bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.(a) -
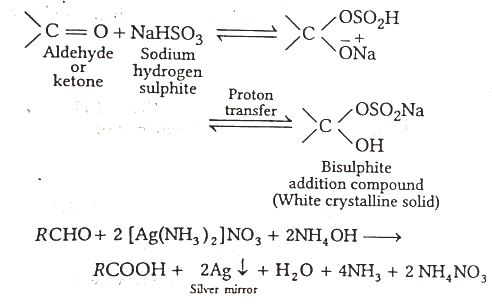
When liquid 'A' is treated with a freshly prepared ammoniacal silver nitrate solution, it gives bright silver mirror. The liquid forms a white crystalline the solid on treatment with sodium hydrogensulphite. Liquid 'B' also forms a white crystalline solid with sodium hydrogensulphite but it does not give test with ammoniacal silver nature. Which of the two liquids is aldehyde? Write the chemical equations of these reactions also.
(a)
*****************************************
CBSE 12th Standard Chemistry Subject Aldehydes , Ketones and Carboxylic Acids Ncert Exemplar 2 Mark Questions 2021 Answer Keys
-
(i) Add I2 and NaOH. P-2- one will give yellow ppt of iodoform, whereas pentan-3- one will not react.
(ii) Add I2 and NaOH. Ethanal will yellow precicipate of iodoform, whereas proponal does not react. -
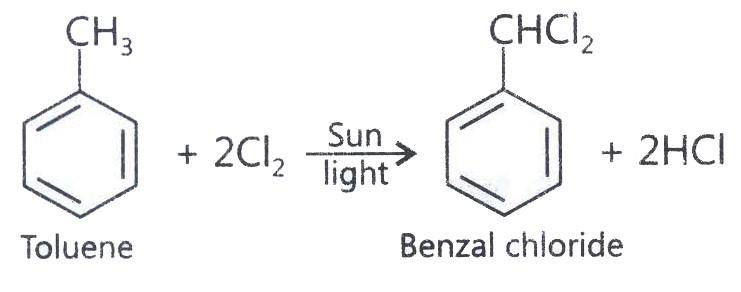
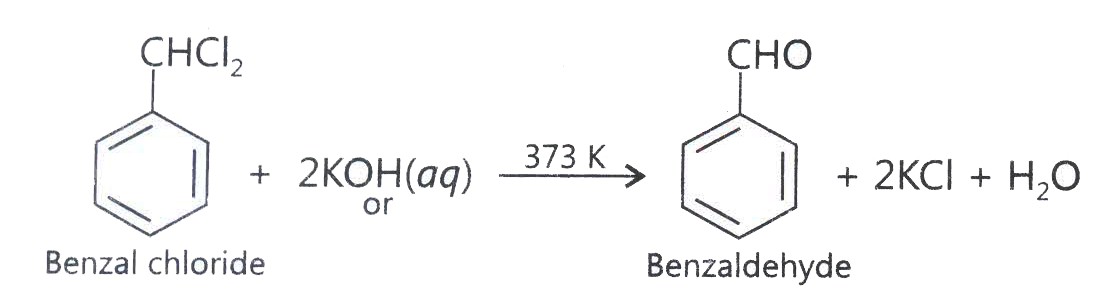
-
'A' is carboxylic acid, 'B' is alcohol, 'C' is easter.
-
Carbon atom in carbonyl compounds acquires slight positive charge and is attacked by nucleophile.
Alkenes undergo electrophilic addition whereas aldehydes and ketones undergo nucleophilic addition because in alkenes the double bond joins two carbon atoms and there is no resultant polarity. -
Since, the liquid A reduces ammoniacal silver nitrate (Tollen's reagent), hence A is aldehyde and B does not give test with ammnoniacal silver nitrate, thus, B is a ketone.
Further, B forms a white crystalline solid on treatment with sodium hydrogen sulphite. This suggests that B is a methyl ketone.