CBSE 12th Standard Chemistry Subject Haloalkanes and Haloarenes Ncert Exemplar 2 Mark Questions With Solution 2021
By QB365 on 25 May, 2021
QB365 Provides the updated NCERT Exemplar Questions for Class 12, and also provide the detail solution for each and every NCERT Exemplar questions. NCERT Exemplar questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 12th Standard Chemistry Subject Haloalkanes and Haloarenes Ncert Exemplar 2 Mark Questions With Solution 2021
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Which of the compounds will react faster in SN1 reaction with the -OH ion?
CH3-CH2-Cl or C6H5-CH2-Cl(a) -
Why iodoform has appreciable antiseptic property?
(a) -
Discuss the role of Lewis acid in the preparation of aryl bromides and chlorides in the dark.
(a)
*****************************************
CBSE 12th Standard Chemistry Subject Haloalkanes and Haloarenes Ncert Exemplar 2 Mark Questions With Solution 2021 Answer Keys
-
SN1 reactions occur through carbocation intermediates. C6H5 -CH2CI readily undergoes ionization to give C6HsCH+2 which is stabilized by resonance.

In constant, CH3CH2CI does not undergo ionization to give CH3CH 2+.As a result, C6H5CH2CI reactsfaster than CH3CH2CI with OH- ion. -
The antiseptic property of iodoform is due to liberation of 12 when it comes in contact with skin and not due to iodoform itself.
\(\underset { Iodo\ form }{ { CHI }_{ 3 } } \overset { contact\ with\ skinow }{ \longrightarrow } \underset { Iodine }{ { I }_{ 2 } } (has\ antiseptic\ property)\) -
The role of Lewis acids in the preparation of aryl chlorides and bromides is to generate the electrophiles, i.e., CI+and Br+ from the corresponding halogens
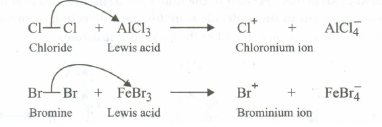
These electrophiles then react with arenes to form the corresponding aryl halides by electrophilic substitution mechanism.






































