CBSE 12th Standard Chemistry Subject Solution Case Study Questions With Solution 2021
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 12 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 12th Standard Chemistry Subject Solution Case Study Questions With Solution 2021
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Read the passage given below and answer the following questions:
The concentration of a solute is very important in studying chemical reactions because it determines how often molecules collide in solution and thus indirectly determine the rate of reactions and the conditions at equilibrium.
There are several ways to express the amount of solute present in a solution. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. Concentration can be expressed in terms of molarity, molality, parts per million, mass percentage, volume percentage, etc.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The molarity (in mol L-1) of the given solution will be(a) 1.56 (b) 1.89 (c) 0.263 (d) 1.44 (ii) Which of the following is correct relationship between mole fraction and molality?
\(\text { (a) } x_{2}=\frac{m M_{1}}{1+m M_{1}}\) \(\text { (b) } x_{2}=\frac{m M_{1}}{1-m M_{1}}\) \(\text { (c) } x_{2}=\frac{1+m M_{1}}{m M_{1}}\) \(\text { (d) } x_{2}=\frac{1-m M_{1}}{m M_{1}}\) (iii) Which of the following is temperature dependent?
(a) Molarity (b) Molality (c) Mole fraction (d) Mass percentage (iv) Which of the following is true for an aqueous solution of the solute in terms of concentration?
(a) 1 M = 1 m (b) 1M > 1m (c) 1M < 1 m (d) Cannot be predicted (a) -
Read the passage given below and answer the following questions:
At 298 K, the vapour pressure of pure benzene, C6H6 is 0.256 bar and the vapour pressure of pure toluene
C6H5CH3 is 0.0925 bar. Two mixtures were prepared as follows:
(i) 7.8 g of C6H6 + 9.2 g of toluene
(ii) 3.9 g of C6H6 + 13.8 g of toluene
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The total vapour pressure (bar) of solution 1 is(a) 0.128 (b) 0.174 (c) 0.198 (d) 0.258 (ii) Which of the given solutions have higher vapour pressure?
(a) I (b) II (c) Both have equal vapour pressure (d) Cannot be predicted (iii) Mole fraction of benzene in vapour phase in solution 1 is
(a) 0.128 (b) 0.174 (c) 0.734 (d) 0.266 (iv) Solution I is an example of a/an
(a) ideal solution (b) non-ideal solution with positive deviation (c) non-ideal solution with negative deviation (d) can't be predicted (a) -
Read the passage given below and answer the following questions:
An ideal solution may be defined as the solution which obeys Raoult's law exactly over the entire range of concentration. The solutions for which vapour pressure is either higher or lower than that predicted by Raoult's law are called non-ideal solutions.
Non-ideal solutions can show either positive or negative deviations from Raoult's law depending on whether the A-B interactions in solution are stronger or weaker than A - A and B - B interactions.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Which of the following solutions is/are ideal solution(s)?
(i) Bromoethane and iodoethane (ii) Acetone and chloroform
(iii) Benzene and acetone (iv)n-heptane and n-hexane(a) only 1 (b) I and II (c) II and III (d) I and IV (ii) Which of the following is not true for positive deviations?
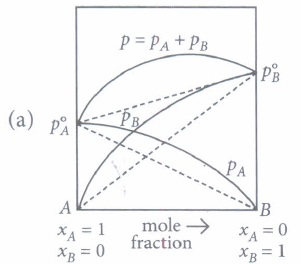
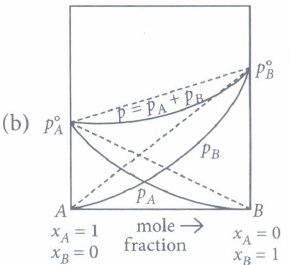
(a) The A-B interactions in solution are weaker than the A -A and B -B interactions. (b) \(P_{A}<P_{A}^{\circ} x_{A} \text { and } P_{B}<P_{B}^{\circ} x_{B}\) (c) Carbon tetrachloride and chloroform mixture is an example of positive deviations. (d) All of these (iii) For water and nitric acid mixture which of the given graph is correct?
(C) Both of these (d) None of these (iv) Water- HCl mixture
I. shows positive deviations II. forms minimum boiling azeotrope
III. shows negative deviations IV. forms maximum boiling azeotrope(a) I and II (b) II and III (c) I and IV (d) III and IV (a) -
Read the passage given below and answer the following questions:
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 20°C is 17.5 mm of Hg.)
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Relative lowering of vapour pressure for the given solution is(a) 0.00348 (b) 0.061 (c) 0.122 (d) 1.75 (ii) The vapour pressure (mm of Hg) of solution will be
(a) 17.5 (b) 0.61 (c) 17.439 (d) 0.00348 (iii) Mole fraction of sugar in the solution is
(a) 0.00348 (b) 0.9965 (c) 0.061 (d) 1.75 (iv) The vapour pressure (mm of Hg) of water at 293 K when 25 g of glucose is dissolved in 450 g of water is
(a) 17.2 (b) 17.4 (c) 17.120 (d) 17.02 (a) -
Read the passage given below and answer the following questions:
Few colligative properties are:
(a) relative lowering of vapour pressure: depends only on molar concentration of solute (mole fraction) and independent of its nature.
(b) depression in freezing point: it is proportional to the molal concentration of solution.
(c) elevation of boiling point: it is proportional to the molal concentration of solute.
(d) osmotic pressure: it is proportional to the molar concentration of solute.
A solution of glucose is prepared with 0.052 g at glucose in 80.2 g of water. (Kf = 1.86 K kg mol-1 and Kb = 5.2 K kg mol-1)
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Molality of the given solution is(a) 0.0052 m (b) 0.0036 m (c) 0.0006 m (d) 1.29 m (ii) Boiling point for the solution will be
(a) 373.05 K (b) 373.15 K (c) 373.02 K (d) 372.98 K (iii) The depression in freezing point of solution will be
(a) 0.0187 K (b) 0.035 K (c) 0.082 K (d) 0.067 K (iv) Mole fraction of glucose in the given solution is
(a) 6.28 x 10-5 (b) 1.23 x 10-4 (c) 0.00625 (b) 0.00028 (a)
*****************************************
CBSE 12th Standard Chemistry Subject Solution Case Study Questions With Solution 2021 Answer Keys
-
(i) (d) : Density of solution = 1.202 g/mL
Volume of solution = \(\frac{100 \mathrm{~g}}{1.202 \mathrm{~g} / \mathrm{mL}}=83.2 \mathrm{~mL}\)
Molarity = \(\frac{n_{\mathrm{KI}}}{\text { Volume of solution in } \mathrm{L}}\)
\(=\frac{0.120 \mathrm{~mol}}{0.0832 \mathrm{~L}}=1.4423 \mathrm{~mol} \mathrm{~L}^{-1}\)
(ii) (a): \(x_{2}=\frac{n_{2}}{n_{1}+n_{2}} ; x_{1}=\frac{n_{1}}{n_{1}+n_{2}} ; \frac{x_{2}}{x_{1}}=\frac{n_{2}}{n_{1}}\)
\(\frac{x_{2}}{x_{1}}=\frac{m_{2} / M_{2}}{m_{1} / M_{1}}=\frac{m_{2}}{m_{1}} \times \frac{M_{1}}{M_{2}}\) ...(i)
Molality = \(\frac{n_{2}}{m_{1}}=\frac{m_{2}}{M_{2} \times m_{1}}\) ...(ii)
From(i) and (ii), m = \(\frac{x_{2}}{x_{1}} \times \frac{1}{M_{1}} ; x_{1}=1-x_{2}\)
Hence. x2 = \(\frac{m M_{1}}{1+m M_{1}}\)
(iii) (a) : Mass does not depend on temperature while volume does. Hence, molarity depends on temperature.
(iv) (b): 1M solution contains 1 mole of solute in less than 1000 g of the solvent whereas 1 m solution has 1 mole of the solute in 1000 g of the solvent. -
(i) (b) : Moles of C6H6 = \(\frac{7.8}{78}=0.1\)
Mole C6H5CH3 = \(\frac{9.2}{92}=0.1\)
Mole fraction of C6H6 = \(\frac{0.1}{0.1+0.1}=0.5\)
=> Mole fraction of C6H5CH3 = 0.5
Vapour pressure of toluene = Vapour pressure of pure toluene x mole fraction of toluene
= 0.0925 x 0.5 = 0.04625
Vapour pressure of benzene = 0.256 x 0.5 = 0.128
Total vapour pressure of solution = 0.17425
(ii) (a) : Moles of benzene in solution-II = \(\frac{3.9}{78}=0.05\)
Moles of toluene in solution-II = \(\frac{13.8}{92}=0.15\)
Vapour pressure of solution
= 0.256 x 0.05 + 0.0925 x 0.15
= 0.0128 + 0.013875 = 0.026675
(iii) (c) : Mole fraction of benzene in vapour phase
\(y_{\text {benzene }}=\frac{p_{\text {benzene }}}{P_{\text {total }}}=\frac{0.128}{0.17425}=0.734\)
(iv) (a) : Benzene and toluene form an ideal solution. -
(i) (d) : II represents negative deviations and III represents positive deviations.
(ii) (b): For positive deviations \(p_{A}>p_{A}^{\circ} x_{A} \text { and } p_{B}>p_{B}^{\circ} x_{B}\)
(iii) (b): Water and nitric acid mixture shows negative deviations from Raoult's law, hence \(p_{A}<p_{A}^{\circ} x_{A} \text { and } p_{B}<p_{B}^{\circ} x_{B}\)
(iv) (d): Water-HCl mixture shows negative deviations from Raoult's law and solutions showing negative deviations from ideal behaviour form maximum boiling azeotrope. -
(i) (a) : Vapour pressure of water \(\left(p_{A}^{\circ}\right)\) = 17.5 mm of Hg
Lowering of vapour pressure \(\left(p_{A}^{\circ}-p_{A}\right)\)= 0.061
Relative lowering of vapour pressure
\(=\frac{p_{A}^{\circ}-p_{A}}{p_{A}^{\circ}}=\frac{0.061}{17.5}=0.00348\)
(ii) (c): P = Vapour pressure of solvent - lowering in vapour pressure = 17.5 - 0.061 = 17.439 mm of Hg
(iii) (a): \(\frac{p_{A}^{\circ}-p_{A}}{p_{A}^{\circ}}=x_{B}=0.00348\)
Hence, mole fraction of sugar = 0.00348
(iv) (b): \(\frac{p_{A}^{\circ}-p_{A}}{p_{A}^{\circ}}=x_{B}=\frac{w_{B} \times M_{A}}{M_{B} \times w_{A}}\)
\(\frac{17.5-p_{A}}{17.5}=\frac{25 \times 18}{450 \times 180}=5.56 \times 10^{-3}\)
\(17.5-p_{A}=17.5 \times 5.56 \times 10^{-3}\)
\(17.5-p_{A}=0.0973\)
P = 17.40 mm Hg -
(i) (b): m \(=\frac{0.052}{180} \times \frac{1000}{80.2}=0.0036\)
(ii) (c): \(\Delta T_{b}=K_{b} \times m=5.2 \times 0.0036=0.0187 \mathrm{~K}\)
\(T_{b}=373+0.0187=373.0187 \mathrm{~K} \approx 373.02 \mathrm{~K}\)
(iii) (d): \(\Delta T_{f}=K_{f} \times m=1.86 \times 0.0036=0.067 \mathrm{~K}\)
(iv) (a): Moles of glucose \(=\frac{0.052}{180}=0.00028\)
Moles 0f water = \(\frac{80.2}{18}=4.455\)
Mole fraction of glucose = \(\frac{0.00028}{4.45+0.00028}=6.28 \times 10^{-5}\)






































