CBSE 12th Standard Physics Subject Dual Nature of Radiation and Matter Chapter Case Study Questions With Solution 2021
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 12 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 12th Standard Physics Subject Dual Nature of Radiation and Matter Case Study Questions With Solution 2021
12th Standard CBSE
-
Reg.No. :
Physics
-
If we allow radiations of a fixed frequency to fall on plate and the accelerating potential difference between the two electrodes is kept fixed, then the photoelectric current is found to increase linearly with the intensity of incident radiation. Here, radiation pressure is P = \(\left(\frac{1+e}{C}\right) I\). As, atmosphere pressure at sea level is 105Pa. If the intensity of light of a given wavelength, is increased, there is an increase in the number of photons incident on a given area in a given time. But the energy of each photon remain the same.
(i) The number of photons hitting the cone second\(\text { (a) } \pi R^{2} I / 2 E\) \(\text { (b) } 2 \pi R^{2} I / E\) \(\text { (c) } \pi R^{2} I / 4 E\) \(\text { (d) } \pi R^{2} I / E\) (ii) A radiation of energy E falls normally on a perfect reflecting surface. The momentum transferred to the surface is
\(\text { (a) } \frac{E}{c}\) \(\text { (b) } \frac{2 E}{c}\) \(\text { (c) } E c\) \(\text { (d) } \frac{E}{c^{2}}\) (iii) Which one is correct?
\(\text { (a) } E^{2}=p^{2} c^{2}\) \(\text { (b) } E^{2}=p^{2} c\) \(\text { (c) } E^{2}=p^{2}\) \(\text { (d) } E^{2}=\frac{p^{2}}{c^{2}}\) (iv) The incident intensity on a horizontal surface at sea level from the Sun is about 1 k W m-2. Assuming that 50% of this intensity is reflected and 50% is absorbed, determine the radiation pressure on this horizontal surface.
(a) 8.2 x 10-2 Pa (b) 5 x 10-6 Pa (c) 3 x 10-5 Pa (d) 6 x 10-5 Pa (v) Find the ratio of radiation pressure to atmospheric pressure P0 about 1 x 105 Pa at sea level.
(a) 5 x 10-11 (b) 4 x 10-8 (c) 6 x 10-12 (d) 8 x 10-11 (a) -
According to de-Broglie, a moving material particle sometimes acts as a wave and sometimes as a particle or a wave associated with moving material particle which controls the particle in every respect. The wave associated with moving particle is called matter wave or de-Broglie wave where wavelength called de-Broglie wavelength is given by \(\lambda=\frac{h}{m v}\)
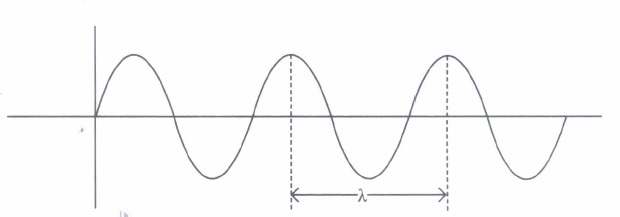
(i) If a proton and an electron have the same de Broglie wavelength, then(a) kinetic energy of electron < kinetic energy of proton (b) kinetic energy of electron = kinetic energy of proton (c) momentum of electron = momentum of proton (d) momentum of electron < momentum of proton (ii) Which of these particles having the same kinetic energy has the largest de Broglie wavelength?
(a) Electron (b) Alpha particle (c) Proton (d) Neutron (iii) Two particles A1 and A2 of masses m1 m2 (m1 > m2) have the same de Broglie wavelength. Then
(a) their momenta are the same. (b) their energies are the same. (c) momentum of A1 is less than the momentum of A2. (d) energy of A1 is more than the energy of A2. (iv) When the velocity of an electron increases, its de Broglie wavelength
(a) increases (b) decreases (c) remains same (d) may increase or decrease (v) Proton and a-particle have the same de-Broglie wavelength. What is same for both of them?
(a) time period (b) energy (c) frequency (d) momentum (a) -
According to wave theory, the light of any frequency can emit electrons from metallic surface provided the intensity of light be sufficient to provided necessary energy for emission of electrons, but according to experimental observations, the light of frequency less than threshold frequency can not emit electrons; whatever be the intensity of incident light. Einstein also proposed that electromagnetic radiation is quantised.If photoelectrons are ejected from a surface when light of wavelength \(\lambda\) 1= 550 nm is incident on it. The stopping potential for such electrons is Vs = 0.19 V. Suppose the radiation of wavelength \(\lambda\)2= 190 nm is incident on the surface.
(i) Photoelectric effect supports quantum nature oflight because
(A) there is a minimum frequency of light below which no photoelectrons are emitted.
(B) the maximum K.E. of photoelectric depends only on the frequency of light and not on its intensity.
(C) even when the metal surface is faintly illuminated, the photo electrons leave the surface immediately.
(D) electric charge of the photoelectrons is quantized.(a) A,B,C (b) B,C (c) C,D (d) A,D,C (ii) In photoelectric effect, electrons are ejected from metals, if the incident light has a certain minimum
(a) wavelength (b) frequency (c) amplitude (d) angle of incidence (iii) Calculate the stopping potential VS2of surface.
(a) 4.47 (b) 3.16 (c) 2.76 (d) 5.28 (iv) Calculate the work function of the surface.
(a) 3.75 (b) 2.07 (c) 4.20 (d) 3.60 (v) Calculate the threshold frequency for the surface.
(a) 500 x 1012 Hz (b) 480 x 1013 Hz (c) 520 x 1011Hz (d) 460 x 1013 Hz (a) -
The photon picture of electromagnetic radiations and the characteristic properties of photons are as follows:In the interaction of radiation with matter, radiation behaves as if it is made of particles like photons. Each photon has energy \(E(=h v=h c / \lambda)\) and momentum \(p\left(=\frac{h v}{c}=\frac{h}{\lambda}\right)\) where h is Planck's constant, v and A are the frequency and wavelength of radiation and c is the velocity of light. The photon energy is independent of the intensity of radiations. All the photons emitted from a source of radiations travel through space with the same speed c. The frequency of photon gives the radiation, a definite energy (or colour) which does not change when photon travels through different media. Photons are not deflected by electric and magnetic fields. This shows that photons are electrically neutral.
(i) Which one among the following shows particle nature of light?(a) Photoelectric effect (b) Interference (c) Refraction (d) Polarization (ii) Which of the following statements about photon is incorrect?
(a) Photons exert no pressure (b) Momentum of photon is \(\frac{h v}{c}\) (c) Rest mass of photon is zero (d) Energy of photon is hv (iii) The rest mass of photon is
\(\text { (a) } \frac{h v}{c}\) \(\text { (b) } \frac{h v}{c^{2}}\) \(\text { (c) } \frac{h v}{\lambda}\) (d) zero (iv) In a photon-particle collision (such as photon-electron collision), which of the following may not be conserved?
(a) Total energy (b) Number of photons (c) Total momentum (d) Both (a) and (b) (v) 'n' photons of wavelengt '\(\lambda\)' are absorbed by a black body of mass 'm'. The momentum gained by the body is
\(\text { (a) } \frac{h}{m \lambda}\) \(\text { (b) } \frac{m n h}{\lambda}\) \(\text { (c) } \frac{n h}{m \lambda}\) \(\text { (d) } \frac{n h}{\lambda}\) (a) -
To study photoelectric effect, an emitting electrode C of a photosensitive material is kept at negative potential and collecting electrode A is kept at positive potential in an evacuated tube. When light of sufficiently high frequency falls on emitting electrode, photoelectrons are emitted which travel directly to collecting electrode and hence an electric cusrent called photoelectric current starts flowing in the circuit, which is directly proportional to the number of photoelectrons emitted by emitting electrode C.
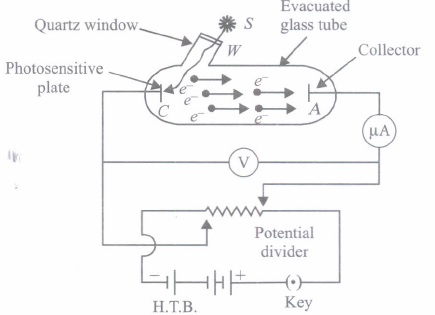
While demonstrating the existence of electromagnetic waves, Hertz found that high voltage sparks passed across the metal electrodes of the detector loop more easily when the cathode was illuminated by ultraviolet light from an arc lamp. The ultraviolet light falling on the metal surface caused the emission of negatively charged particles,which are now known to be electrons, into the surrounding space and hence enhanced the high voltage sparks.
(i) Cathode rays were discovered by(a) Maxwell Clerk James (b) Heinrich Hertz (c) William Crookes (d) J. J. Thomson (ii) Cathode rays consists of
(a) photons (b) electrons (c) pistons (d) a-particles. (iii) Who discovered the charge on an electron for the frist time?
(a) Millikan (b) Thomson (c) Kelvin (d) Coulomb (iv) The dual nature of light is exhibited by
(a) diffraction and photoelectric effect (b) photoelectric effect (c) refraction and interference (d) diffraction and reflection (v) In the phenomenon of electric discharge through gases at low pressure, the coloured glow in the tube appears as a result of
(a) collisions between the charged particles emitted from the cathode and the atoms of the gas (b) collision between different electrons of the atoms of the gas (c) excitation of electrons in the atoms (d) collision between the atoms of the gas. (a)
*****************************************
CBSE 12th Standard Physics Subject Dual Nature of Radiation and Matter Case Study Questions With Solution 2021 Answer Keys
-
(i) (d): Power of light received by the cone \(=I\left(\pi R^{2}\right)\)
Let number of photons hitting the cone per second is n.
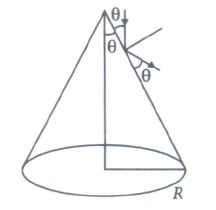
Then, \(n E=I \pi R^{2} \Rightarrow n=\pi R^{2} I / E\)
(ii) (b): Initial moment \(p_{i}=\frac{E}{c}\)
For a perfectly selecting surface
Final momentum \(p_{f}=\frac{-E}{c}\)
\(\Delta p=p_{f}-p_{i}=\frac{-E}{c}-\frac{E}{c}=\frac{-2 E}{c}\)
Hence a momentum \(\frac{2 E}{c}\) is transferred to the reflecting surface.
(iii) (a): According to the theory of relatively,
\(E=m c^{2}=m c . c=p c\)
\(\text { or } \quad E^{2}=p^{2} c^{2}\)
where p is the momentum of a photon.
(iv) (b): I = 1 kWm-2
As, 50% of light is reflected, thus e = 0.5
Radiation pressure \(P=\frac{(1+e) I}{c}\)
\(\therefore \quad P=\frac{(1+0.5) \times 1000}{3 \times 10^{8}}=5 \times 10^{-6} \mathrm{~Pa}\)
(v) (a) : \(\frac{P_{\mathrm{rad}}}{P_{0}}=\frac{5 \times 10^{-6}}{1 \times 10^{5}}=5 \times 10^{-11}\) -
(i) (c) :de Broglie wavelength, \(\lambda=\frac{h}{p}\)
where p is the momentum of the particle For electron \(\lambda_{e}=\frac{h}{p_{e}}\)
For proton \(\lambda_{e}=\frac{h}{p_{p}}\)
\(\text { As } \lambda_{e}=\lambda_{p} \Rightarrow p_{e}=p_{p}\) (Given)
or Momentum of electron = Momentum of proton
(ii) (a) : \(\text { As } \lambda-\frac{h}{\sqrt{2 m K}} \text { so } \lambda \sim \frac{1}{\sqrt{m}}\)
Out of the given particles m is least for electron, therefore electron has the largest value of de Broglie wavelength.
(iii) (a) : \(\text { As } \lambda=\frac{h}{p} \text { or } p=\frac{h}{\lambda} \text { or } p \propto \frac{1}{\lambda}\)
\(\therefore \quad \frac{P_{1}}{P_{2}}=\frac{\lambda_{2}}{\lambda_{1}}=\frac{\lambda}{\lambda}=1 \text { or } p_{1}=p_{2} \)
\(\text { Also } E=\frac{1}{2} \frac{p^{2}}{m}=\frac{1}{2 m} \frac{h^{2}}{\lambda^{2}} \quad\left(\because p=\frac{h}{\lambda}\right) \)
\((\text { or } \quad E \propto \frac{1}{m} \therefore \frac{E_{1}}{E_{2}}=\frac{m_{2}}{m_{1}}<1 \text { or } E_{1} )\)
(iv) (b): The de Broglie wavelength is given by
\(\lambda=\frac{h}{p}=\frac{h}{m v}\)
So if the velocity of the electron increases, the de -Broglie wavelength decreases.
(v) (d): \(\lambda=\frac{h}{p}\) when \(\lambda\) is same, P is also same. -
(i) (a): The existence of the frequency and the instantaneous emission of photo electrons support the quantum nature of light.
(ii) (b): For photoelectric emission, the incident light must have a certain minimum frequency, called threshold frequency.
(iii) (a): From Einstein's relation
\(e V_{s}=h v-W\)
As work function is a constant for a surface
\(e\left(V_{s_{2}}-V_{s_{1}}\right)=h\left(v_{2}-v_{1}\right)\)
\(V_{s_{2}}=V_{s_{1}}+\frac{h}{e}\left(v_{2}-v_{1}\right)\)
\(=0.19+1240\left(\frac{1}{190}-\frac{1}{550}\right)=4.47 \mathrm{~V}\)
(iv) (b): \(W=\frac{h c}{\lambda_{1}}-e V_{s_{1}}=\frac{1240}{550}-0.19=2.07 \mathrm{eV}\)
(v) (a): hve = W -
(i) (a): Particle nature of light was established by photoelectric effect.
(ii) (a): Photons move with velocity of light and have energy hv. Therefore, they also exert pressure.
(iii) (d): The rest mass of photon is zero.
(iv) (b): In a photon-particle collision, (such as photon electron collision), the total energy and total momentum are conserved. However, the number of photons may not be conserved in photon-particle collision. The photon may be absorbed or a new photon may be created.
(v) (d): Energy of n photons \(E=\frac{n h c}{\lambda}\)
Momentum gam. ed by the body, \(p=\frac{E}{c}=\frac{n h c}{\lambda c}=\frac{n h}{\lambda}\) -
(i) (c)
(ii) (b)
(iii) (a)
(iv) (a)
(v) (c): In discharge tube, collision between charged particles emitted from cathode and atoms of the gas results to colorless glow in the tube.






































