Class 11th Chemistry - Redox Reactions Case Study Questions and Answers 2022 - 2023
By QB365 on 09 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 11 Chemistry Subject - Redox Reactions, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Redox Reactions Case Study Questions With Answer Key
11th Standard CBSE
-
Reg.No. :
Chemistry
-
Redox reactions are reactions in which oxidation and reduction takes place simultaneously. Oxidation number are assigned in accordance with the set of rules. Oxidation number and ion electron methods both are used in balancing ionic equations. Redox reactions are classified as combination, decomposition, displacement and disproportionation reactions. The concept of redox couple and electrode processes is basis of electrolysis and electrochemical cells.
(a) What are oxidation number of each individual Br in Br3 O8 ?
(b) If electrolysis of CuSO4 solution is carried out using Cu electrodes, what will be reaction taking place at anode.
(c) What is oxidation number of Cr in CrO5?
(d) Give one example of disproportionation reaction.(a) -
Redox reactions are important class of reactions which are taking place in our daily life. Metals are good reducing agents because they can lose electrons easily whereas non-metals are good oxidising agents which can gain electrons easily. In electrolytic cells, electricity is passed to bring about redox reaction. All rechargeable batteries act as electrolytic cells while recharging. Electrochemical cells produce electricity as a result of redox reaction. Salt bridge is used in electrochemical cell to complete internal circuit and prevents accumulation of charges.
(a) What is electrochemical cell?
(b) Why is anode called oxidation electrode?
(c) Give one example of rechargeable cells widely used in vehicles.
(d) Highly reactive metals are obtained by electrolysis of their molten ores, why?
(e) What is direction of flow of current and electrons?
(f) What is standard electrode potential?
(g) What is meaning of -ve value of reduction potential? Give example.(a) -
The standard electrode potentials at 298 K
Ions are present as aqueous species and H 2O as liquid; gases and solids are shown by g and s respectively.Reaction (Oxidised form + ne- \(\rightarrow\) Reduced form) \(\mathbf{E}^{\odot} / \mathbf{V}\) \(\mathbf{F}_{2}(g)+2 e^{-} \longrightarrow 2 \mathbf{F}\)
\(\mathrm{Co}^{3+}+e^{-} \longrightarrow \mathrm{Co}^{2+}\)
\(\mathbf{H}_{2} \mathbf{O}_{2}+\mathbf{2} \mathbf{H}^{+}+\mathbf{2} e^{-} \longrightarrow \mathbf{2} \mathbf{H}_{2} \mathbf{O}\)
\(\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 e^{-} \longrightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_{2} \mathrm{O}\)
\(\mathbf{A u}^{3+}+\mathbf{3} \boldsymbol{e}^{-} \longrightarrow \mathbf{A u}(s)\)
\(\mathrm{Cl}_{2}(g)+2 e^{-} \longrightarrow 2 \mathrm{Cl}^{-}\)
\(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+\mathbf{1 4 H}^{+}+6 e^{-} \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_{2} \mathrm{O}\)
\(\mathrm{O}_{2}(\mathrm{~g})+4 \mathrm{H}^{+}+4 e^{-} \longrightarrow 2 \mathrm{H}_{2} \mathrm{O}\)
\(\mathrm{MnO}_{2}(s)+4 \mathrm{H}^{+}+2 e^{-} \longrightarrow \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O}\)
\(\mathbf{B r}_{2}+2 e^{-} \longrightarrow 2 \mathbf{B r}^{-}\)
\(\mathrm{NO}_{3}^{-}+4 \mathrm{H}^{+}+3 e^{-} \longrightarrow \mathrm{NO}+\underline{2} \mathrm{H}_{2} \mathrm{O}\)
\(2 \mathrm{Hg}^{2+}+2 e^{-} \longrightarrow \mathrm{Hg}_{2}^{2+}\)
\(\mathbf{A g}^{+}+\boldsymbol{e}^{-} \longrightarrow \mathbf{A g}(\boldsymbol{s})\)
\(\mathbf{F e}^{3+}+e^{-} \longrightarrow \mathbf{F e}^{2+}\)
\(\mathrm{O}_{2}(g)+2 \mathrm{H}^{+}+2 e^{-} \longrightarrow \mathbf{H}_{2} \mathrm{O}_{2}\)
\(\mathbf{I}_{2}(s)+2 e^{-} \longrightarrow 2 \mathbf{I}^{-}\)
\(\mathbf{C u}^{+}+e^{-} \longrightarrow \mathbf{C u}(\boldsymbol{s})\)
\(\mathrm{Cu}^{2+}+2 e=\mathrm{Cu}(s)\)
\(\mathbf{A g C l}(s)+e^{-} \longrightarrow \mathbf{A g}(s)+\mathbf{C l}\)
\(\mathbf{A g B r}(s)+e^{-} \longrightarrow \mathbf{A g}(s)+\mathbf{B r}^{-}\)
\(2 \mathrm{H}^{+}+2 e^{-} \longrightarrow \mathbf{H}_{2}(g)\)
\(\mathbf{P b}^{2+}+2 e^{-} \longrightarrow \mathbf{P b}(s)\)
\(\mathrm{Sn}^{2+}+2 e^{-} \longrightarrow \operatorname{Sn}(s)\)\(\)\(\)
\(\)\(\mathbf{N i}^{2+}+2 e^{-} \longrightarrow \mathbf{N i}(s)\)
\(\)\(\mathrm{Fe}^{2+}+2 e-\mathrm{Fe}(s)\)
\(\)\(\mathrm{Cr}^{3+}+3 e^{-} \longrightarrow \mathrm{Cr}(s)\)
\(\)\(\mathbf{Z n}^{2+}+2 e^{-} \longrightarrow \mathbf{Z n}(s)\)
\(\)\(\mathbf{2} \mathbf{H}_{2} \mathbf{O}+\mathbf{2} e^{-} \longrightarrow \mathbf{H}_{2}+\mathbf{2} \mathbf{O H}^{-}\)
\(\)\(\mathbf{A l}^{3+}+\mathbf{3 e}^{-} \longrightarrow \mathbf{A l}(\boldsymbol{s})\)
\(\)\(\mathbf{M g}^{2+}+2 e^{-} \longrightarrow \mathbf{M g}(s)\)
\(\)\(\)\(\mathrm{Na}^{+}+e^{-} \longrightarrow \mathrm{Na}(s)\)
\(\mathrm{Ca}^{2+}+2 e^{-} \longrightarrow \mathrm{Ca}(s)\)
\(\mathbf{K}^{+}+e^{-} \longrightarrow \mathbf{K}(\boldsymbol{s})\)
\(\mathbf{L i}^{+}+e^{-} \longrightarrow \mathbf{L i}(s)\)2.87 1.81 1.78 1.51 1.40 1.36 1.33 1.23 1.23 1.09 0.97 0.92 0.80 0.77 0.68 0.54 0.52 0.34 0.22 0.10 0.00 0.13 0.14 0.25 0.44 0.74 0.76 0.83 1.66 2.36 2.71 2.87 2.93 3.05 1. A negative \(\boldsymbol{E}^{\ominus}\) means that the redox couple is a stronger reducing agent than the H+/H2 couple.
2. A positive \(\boldsymbol{E}^{\ominus} \) means that the redox couple is a weaker reducing agent than the H+/H2 couple.
(a) Which is best oxidising agent and why?
(b) Which is best reducing agent and why?
(c) Calculate \(\mathbf{E}^{\circ} \text { cell }\) lof Zn(s)/Zn2+(1M) II Cu2 +(aq)/Cu(s).
(d) If a redox couple has negative Eo, is it stronger or weaker reducing agent than H+/H2 couple?
(e) Arrange K, Ag, Hg, Mg, Cr in increasing order of reducing power.
(f) Arrange AI, Cu, Fe, Mg and Zn in order in which they will displace each other from salt solution.(a)
Case Study
*****************************************
Answers
Redox Reactions Case Study Questions With Answer Key Answer Keys
-
(a)
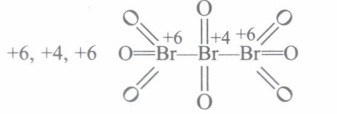
(b) Cu \(\rightarrow\) Cu 2 + + 2e-
(c)
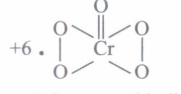
\(\therefore\) It has peroxide linkage.
(d) 2Cu + \(\rightarrow\) Cu2+ + Cu
(e) \(\begin{aligned} \mathrm{MnO}_{4}^{2-} & \longrightarrow \mathrm{MnO}_{4}^{-}+e^{-} \\ 2 e^{-}+4 \mathrm{H}^{+}+\mathrm{MnO}_{4}^{2-} & \longrightarrow \mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O} \\ \hline 3 \mathrm{MnO}_{4}^{2-}+4 \mathrm{H}^{+} & \longrightarrow 2 \mathrm{MnO}_{4}^{-}+\mathrm{MnO}_{2}+2 \mathrm{H}_{2} \mathrm{O} \\ \hline \end{aligned}\) -
(a) The cell in which chemical energy of redox reaction is converted into electrical energy.
(b) It is because loss of electrons take place at anode, i.e., oxidation takes place.
(c) Lead storage battery
(d) It is because these metals are good reducing agents, cannot be obtained by chemical reduction.
(e) Electrons flow from anode to cathode where as current flows from cathode to anode.
(f) When concentration of each species is unity, any gas involved is at 1 bar, temperature is 298 K, the potential of electrode is called standard electrode potential measured with respect to standard hydrogen electrode.
(g) It means redox couple is stronger reducing agent than H+/H2 couple.
E.g. \(\mathrm{E}_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\circ}=\) -0.76 V, Zn is stronger reducing agent than H2. -
(a) F2 is best oxidising agent because it has highest standard reduction potential.
(b) Lithium is best reducing agent because it has lowest standard reduction potential.
(c) \(\mathrm{E}_{\text {cell }}^{\circ}=\mathrm{E}^{\circ} \text { cathode }-\mathrm{E}^{\circ} \text { anode }\)
= + 0.34V - (- 0.76V)
= 1.10 V
(d) It will be stronger reducing agent.
(e) Ag < Hg < Cr < Mg < K
(f) Cu, Fe, Zn, AI, Mg is increasing order of reactivity and increasing ability to displace other metals.
Case Study



































