Class 12th Chemsitry - Alcohols , Phenols and Ethers Case Study Questions and Answers 2022 - 2023
By QB365 on 08 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 12 Chemsitry Subject - Alcohols , Phenols and Ethers, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Alcohols , Phenols and Ethers Case Study Questions With Answer Key
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Read the passage given below and answer the following questions:
Although chlorobenzene is inert to nucleophilic substitution, however it gives quantitative yield of phenol when heated with aq. NaOH at high temperature and under high pressure. As far as electrophilic substitution in phenol is concerned the - OH group is an activating group, hence, its presence enhances the electrophilic substitution at o- and p-positions.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Conversion of chlorobenzene into phenol involves(a) modified SN1 mechanism (b) modified SN2 mechanism (c) both (a) and (b) (d) elimination-addition mechanism. (ii) Phenol undergoes electrophilic substitution more readily than benzene because
(a) the intermediate carbo cation is a resonance hybrid of more resonating structures than that from benzene (b) the intermediate is more stable as it has positive charge on oxygen, which can be better accommodated than on carbon (c) in one of the canonical structures, every atom (except hydrogen) has complete octet (d) the -OH group is o, p-directing which like all other o, p-directing group, is activating. (iii) Phenol on treatment with excess of conc. HNO3 gives
(a) o-nitrophenol (b) p-nitrophenol (c) o-and p-nitrophenol (d) 2, 4, 6-trinitrophenol (iv) The major product of the following reaction is
(a) -
Read the passage given below and answer the following questions:
A compound (X) containing C, Hand O is unreactive towards sodium. It also does not react with Schiff's reagent. On refluxing with an excess ofhydroiodic acid, (X) yields only one organic product (Y). On hydrolysis, (Y) yields a new compound (Z) which can be converted into (Y) by reaction with red phosphorus and iodine. The compound (Z) on oxidation with potassium permanganate gives a carboxylic acid. The equivalent weight of this acid is 60.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The compound (X) is an(a) acid (b) aldehyde (c) alcohol (d) ether (ii) The IUPAC name of the acid formed is
(a) methanoic acid (b) ethanoic acid (c) propanoic acid (d) butanoic acid. (iii) Compound (Y) is
(a) ethyl iodide (b) methyl iodide (c) propyl iodide (d) mixture of (a) and (b). (iv) Compound (X) on treatment with excess of Cl2 in presence of light gives
(a) \(\alpha\) - chlorodiethyl ether (b) \(\alpha\), \(\alpha\)' -dichlorodiethyl ether (c) perchlorodiethyl ether (d) none of these. (a) -
Read the passage given below and answer the following questions:
Both alcohols and phenols are acidic in nature, but phenols are more acidic than alcohols. Acidic strength of alcohols mainly depends upon the inductive effect. Acidic strength of phenols depends upon a combination of both inductive effect and resonance effects of the substituent and its position on the benzene ring. Electron withdrawing groups increases the acidic strength of phenols whereas electron donating groups decreases the acidic strength of phenols. Phenol is a weaker acid than carboxylic acid.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Phenols are highly acidic as compare to alcohols due to(a) the higher molecular mass of phenols (b) the stronger hydrogen bonds in phenols (c) alkoxide ion is a strong conjugate base (d) phenoxide ion is resonance stabilised. (ii) The correct decreasing order of pKa value is
(a) II > IV > I > III (b) IV > II > III > I (c) III > II > IV > I (d) IV > I > II > III (iii) The compound that does not liberate CO2, on treatment with aqueous sodium bicarbonate solution is
(a) benzoic acid (b) benzenesulphonic acid (c) salicylic acid (d) carbolic acid. (iv) Most acidic amongst the following is
(a) -
Read the passage given below and answer the following questions:
Ethers are readily cleaved by HI or HBr at 373 K to form an alcohol and an alkyl halide.
\(R-\mathrm{O}-R+\mathrm{HX} \stackrel{373 \mathrm{~K}}{\longrightarrow} R-X+R-\mathrm{OH}\)
\(R-\mathrm{OH}+\mathrm{HX} \stackrel{373 \mathrm{~K}}{\longrightarrow} R-X+\mathrm{H}_{2} \mathrm{O}\)
Mixed ether, containing primary or secondary alkyl group, when heated with hydrogen halide, the lower alkyl group forms halide and higher will form an alcohol. Tertiary alkyl ether when heated with hydrogen halide gives tertiary alkyl halide.
The following questions are multiple choice questions. Choose the most appropriate answer :
(i) Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated HI?(c) CH3-CH2-CH2-CH2-O-CH3 (ii) When CH2=CH-O-CH2-CH3 reacts with one mole of HI, one of the products formed is
(a) ethane (b) ethanol (c) iodoethene (d) ethanal (iii) (CH3)3COCH3 and CH3OC2H5 are treated with hydroiodic acid. The fragments obtained after reactions are respectively
(a) \(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{CI}+\mathrm{CH}_{3} \mathrm{OH} ; \mathrm{CH}_{3} \mathrm{I}+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\)
(b) \(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{CI}+\mathrm{CH}_{3} \mathrm{OH} ; \mathrm{CH}_{3} \mathrm{OH}+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{I}\) (c) \(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{COH}+\mathrm{CH}_{3} \mathrm{I} ; \mathrm{CH}_{3} \mathrm{OH}+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{I}\) (d) \(\mathrm{CH}_{3} \mathrm{I}+\left(\mathrm{CH}_{3}\right)_{3} \mathrm{COH} ; \mathrm{CH}_{3} \mathrm{I}+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) (iv) Which of the following ether is unlikely to be cleaved by hot conc. HBr?
(a) -
Read the passage given below and answer the following questions:
An organic compound (A) having molecular formula C6H6O gives a characteristic colour with aqueous FeCl3 solution. (A) on treatment with CO2 and NaOH at 400 K under pressure gives (B), which on acidification gives a compound (C). The compound (C) reacts with acetyl chloride to give (D) which is a popular pain killer.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Compound (A) is(a) 2-hexanol (b) dimethyl ether (c) phenol (d) 2-methyl pentanol. (ii) Number of carbon atoms in compound (D) is
(a) 7 (b) 6 (c) 8 (d) 9 (iii) The conversion of compound (A) to (C) is known as
(a) Reimer- Tiemann reaction (b) Kolbe's reaction (c) Schimdt reaction (d) Swarts reaction (iv) Compound (A) on heating with compound (C) in presence of POCl3 gives a compound (D) which is used
(a) in perfumery as a flavouring agent (b) as an antipyretic (c) as an analgesic (d) as an intestinal antiseptic. (a) -
Read the passage given below and answer the following questions:
Reimer- Tiemann reaction introduces an aldehyde group, on aromatic ring of phenol, ortho to the hydroxyl group. This is a general method for the synthesis of substituted salicylaldehydes as depicted below.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Reimer-Tiemann reaction is an example of(a) nucleophilic substitution reaction (b) electrophilic substitution reaction (c) nucleophilic addition reaction (d) electrophilic addition reaction (ii) Which of the following reagents is used in the given reaction in steps I?
(a) aq. NaOH + CH3CI (b) aq. NaOH + CH2Cl2 (c) aq. NaOH + CHCl3 (d) aq. NaOH + CCl4 (iii) The structure of the intermediate [A] is
(iv) When phenol reacts with chloroform in presence of KOH, the product formed is
(a) salicylic acid (b) salicylaldehyde (c) both (a) and (b) (d) none of these. (a) -
Read the passage given below and answer the following questions:
Dehydration of alcohols can lead to the formation of either alkenes or ethers. This dehydration can be carried out either with protonic acids such as cone. H2SO4, H3PO4 or catalysts such as anhydrous ZnCl2 or Al2O3. When primary alcohols are heated with cone. H2SO4 at 433-443 K, they undergo intramolecular dehydration to form alkenes. Secondary and tertiary alcohols undergo dehydration under milder conditions. The ease of dehydration of alcohols follows the order: 3° > 2° > 1°. The dehydration of alcohols always occurs in accordance with the Saytzeffs rule. Primary alcohols when heated with protic acid at 413 K, gives dialkyl ether.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Which one of the following alcohols undergoes acid-catalysed dehydration to alkenes most readily?(a) (CH3)2CHCH2OH (b) (CH3)3COH (c) CH3CHOHCH3 (d) CH3CH2CH2OH (ii) Dehydration of alcohol is an example of which type of reaction?
(a) Substitution (b) Elimination (c) Addition (d) Rearrangement (iii) The alcohol which does not give a stable compound on dehydration is
(a) ethyl alcohol (b) methyl alcohol (c) n-propyl alcohol (d) n-butyl alcohol (iv)
products.
The most stable product(s) is/are
(c) both (a) and (b) (d) none of these. (a) -
Read the passage given below and answer the following questions:
Williamson's synthesis is used for the preparation of symmetrical as well as unsymmerical ether. It is SN2 reaction mechanism. In Williamson's synthesis, 1° alkyl halide are used for preparation of ethers because 2° and 3°alkyl halide give alkene: Ethers are cleaved by hydrogen halides to alcohol and alkyl halide where alkyl halide is corresponding to that alkyl which has less number of carbon atom (it is because ofless steric hindrance). In polar media unsymmetrical ether like tertiary butyl ethyl ether gives ethyl alcohol and tertiary butyl halide as reaction proceeds via carbocation.
In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Rate of reaction of alkyl halide in Williamson's synthesis reaction is 1°RX> 2°RX > 3°RX.
Reason: It is a type of bimolecular substitution reaction (SN2).
(ii) Assertion: t-Butyl methyl ether is not prepared by the reaction of t-butyl bromide with sodium methoxide.
Reason: Sodium methoxide is a weak nucleophile.
(iii) Assertion: When isopropyl bromide is treated with sodium isopropoxide, di-isopropyl ether is obtained as a major product. .
Reason: With secondary alkyl halides, both substitution and elimination occur.
(iv) Assertion: Both symmetrical arid unsymmetrical ethers can be prepared by Williamson's synthesis.
Reason: Williamson's synthesis is an example of nucleophilic substitution reaction.(a) -
Read the passage given below and answer the following questions:
Due to intermolecular hydrogen bonding, the boiling points of alcohols and phenols are much higher than those of corresponding haloalkanes, haloarenes, aliphatic and aromatic hydrocarbons. Among isomeric alcohols, the boiling points follow the order : primary > secondary > tertiary. Boiling points of ethers are much lower than those of isomeric alcohols. The solubility of alcohols in water decreases as the molecular mass of alcohols increases. Amongst isomeric alcohols solubility increases with branching. The solubility of phenols in water is much lower than that of alcohols. Lower ethers such as dimethyl ether and ethyl methyl ether are soluble in water, but the solubility decreases as the molecular mass increases.
In these questions (i-iv) a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Alcohols have higher boiling points than ethers of comparable molecular masses.
Reason: Alcohols and ethers are isomeric in nature.
(ii) Assertion: The solubility of phenols in water is much lower than that of alcohols.
Reason: Phenols do not form H-bonds with water.
(iii) Assertion : Among n-butane, ethoxyethane,1-propanol and 2-propanol, the increasing order of boiling points is,1-butanol < 1-propanol < ethoxyethane < n-butane.
Reason: Boiling point increases with increase in molecular mass.
(iv) Assertion: Dimethyl ether and diethylether are soluble in water.
Reason: As the molecular mass increases, solubility of ethers in water decreases.(a) -
Read the passage given below and answer the following questions:
Lucas test is a test to differentiate between primary, secondary and tertiary alcohols. This test consists of treating an alcohol with Lucas reagent, and turbidity, due to the formation of insoluble alkyl chloride, is observed. Lucas test is based on the difference in reacting of three classes of alcohols with hydrogen chloride via SN1 reaction. The different reactivity reflects the differing ease of formation of the corresponding carbocations.
In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Equimolar mixture of conc. HCI and anhydrous ZnCl2 is called Lucas' reagent.
Reason : Lucas' reagent can be used to distinguish between methanol and ethanol.
(ii) Assertion: 2-Methyl-2-butanol gives no turbidity with Lucas' reagent at room temperature.
Reason: It is a 3° alcohol
(iii) Assertion: Amongst the compounds, H2C =CHCH2OH (I), C6H5OH (II), CH3CH2CH2OH (III) and (CH3)3COH (IV), only (IV) reacts with Lucas' reagent at room temperature.
Reason : Tertiary alcohol gives turbidity immediately with Lucas' reagent.
(iv) Assertion: Lucas test can be used to distinguish between 1-propanol and 2-propanol.
Reason : Lucas test is based upon the difference in reactivity of primary, secondary and tertiary alcohols with conc. HCI and anhyd. ZnCI2.(a) -
Alcohols and phenols are most important compounds used in our daily life. Alcohols are prepared by hydration of alkenes, fermentation of glucose, reduction of aldehydes, ketones, carboxylic acids and esters.
Alcohols are soluble in water. Boiling points increase with increase in molar mass and decrease with branching. Alcohols on dehydration gives alkene at 443K, follow carbocation mechanism. Excess of alcohol at 413K on dehydration with cone. H2 SO4 also follow carbocation mechanism but gives diethyl ether. Alcohols- undergo nucleophilic substitution reactions, esterification with carboxylic acids and derivatives like amides, acid halides, acid anhydride.
Phenol is prepared from cumene, diazonium salts, anisole, chlorobenzene. Phenol is used to prepare salicylaldehyde, salicylic acid, aspirin, methyl salicylate, p-benzoquinone. Phenol undergoes electrephilic substitution reaction at o & p-position. Ethers are functional isomers of alcohols, have low boiling points. Ethers are used as solvents. Unsymmetrical ethers are prepared by Williamson synthesis. Ethers react with HI and undergo SN 1 or SN2 mechanism depending upon stability of carbocation formed. Aromatic ethers like anisole undergoes electrophilic substitution at o & p-position.
(a) Write IUPAC name of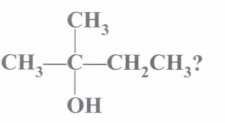
(b) Out of tert, butyl alcohol and n-butanol, which will undergo dehydration faster and why?
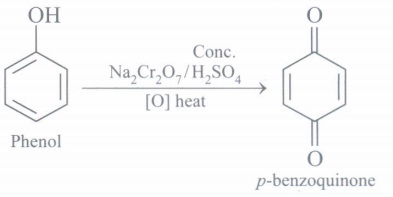
(c) Convert phenol to p-benzoquinone
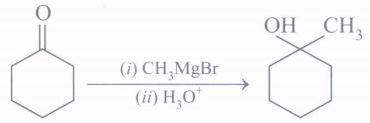
(d) Complete the chemical reaction:
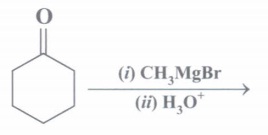
(e) Why is C-OH bond length in CH3OH longer than C-OH bond length in phenol?
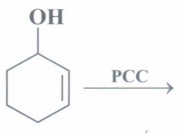
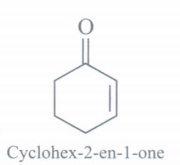
(f) Identify the product:
(g) Why is bond angle in alcohol less than tetrahedral bond angles?(a)
bond angle in alcohol less than tetrahedral bond angles?(a) -
Alcohols play very important role in our daily life. Ordinary sprit used as an antiseptic contains methanol. Ethanol is present in cough syrups, tonics, wine, beer and whisky, Sugar, starch, cellulose are carbohydrates which also contain large number -OH groups. Phenol is also an antiseptic in low concentration (0.2%) where as 2% solution of phenol is used as disinfectant. The fragrance of rose is due to citronellol (unsaturated alcohol). Phenol is used for preparation of many useful compounds like aspirin, methyl salicylate (Iodex) and phenyl salicylate (salol) used as intestinal antiseptic.
(a) How is phenol prepared from cumene? What is advantage of this method?
(b) How is phenol converted into salicylic acid?
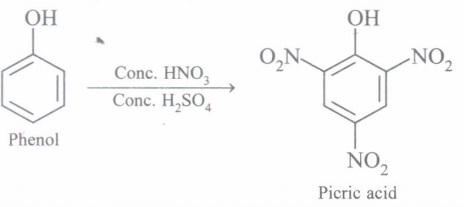
(c) Convert phenol to picric acid
(d) Distinguish between phenol and benzyl alcohol?
(e) Why does phenol turn pink after long standing?(a) -
Observe the following table showing boiling points of alcohol, molar mass. Study the table and answer the questions based on table and related studied concept.
Alcohol Boiling Point Molar Mass CH3OH 64°C 32 g mol-1 C2H5OH 78°C 46 g mol-1 C3H7OH (n-propyl alcohol) 97°C 60 g mol-1 Isopropyl alcohol 82.5oC 60 g mol-1 n-butanol 118°C 74 g mol-1 Isobutyl alcohol 108°C 74 g mol-1 Butan-2-ol 100°C 74 g mol-1 Tert. butyl alcohol 83°C 74 g mol-1 (a) Why do alcohols have higher boiling points than haloalkanes, ethers, aldehydes and ketones?
(b) Why does tertiary butyl alcohol have lower boiling point than n-butyl alcohol?
(c) How does boiling point vary with increase in carbon chain?
(d) How is solubility of alcohol vary with increase in molar mass?
(e) Which alcohol is most acidic and why?(a) -
Table given below has compounds and their pKa values. Study the table and answer the questions based on table and related studied concepts.
Compound pKa Ethanol 15.9 Phenol 9.98 o-cresol 10.28 p-cresol 10.14 m-cresol 10.08 o-nitro phenol 7.23 p-nitro phenol 7.15 m-nitro phenol 8.40 2, 4-dinitro phenol 4.0 Picric acid 0.71 m-methoxy phenol 9.65 o-methoxy phenol 9.96 p-methoxy phenol 10.21 m-amino phenol 9.87 (a) Which phenolic compound is most acidic?
(b) What is relationship between pKa and acidic character?
(c) Why are cresols weaker acids than phenol?
(d) Which has more electron withdrawing effect (-I)-I effect -OCH3 or -NH2? Why?
(e) Why is o-fluoro phenol weakest acid than pand m-fluoro phenol?(a)
Case Study
*****************************************
Answers
Alcohols , Phenols and Ethers Case Study Questions With Answer Key Answer Keys
-
(i) (d)
-
(i) (d): Since the compound X is unreactive towards sodium so it is neither an acid nor an alcohol. Since the compound X is unreactive towards Schiff's base so it is not an aldehyde.
The compound X forms only one product on reaction with excess HI, indicates that the compound X may be ether.
(ii) (b): The reactions can be written as :
Since the equivalent weight of carboxylic acid is 60. So, it must be CH3COOH i.e., ethanoic acid.
(iii) (a) : The alcohol Z in that case should be C2H5OH and the compound Y should be ethyl iodide.
X is therefore diethyl ether (C2H5 - O -C2H5)
(iv) (C): In the presence of light and excess of chlorine, all the hydrogen atoms of diethyl ether are substituted to give perchlorodiethyl ether.
\( \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{O}-\mathrm{CH}_{2} \mathrm{CH}_{3}+10 \mathrm{Cl}_{2} \stackrel{\mathrm{h} \mathrm{v}}{\longrightarrow}\\ \quad\quad\quad \quad \quad \quad \quad \quad \quad \quad \quad { (excess) } \) \( \mathrm{CCl}_{3} \mathrm{CCl}_{2}-\mathrm{O}-\mathrm{CCl}_{2}-\mathrm{CCl}_{3}+10 \mathrm{HCl}\\ \text { Perchlorodiethyl ether } \) -
(i) (d)
(ii) (a) : Weaker acids have higher pKa.
- OCH3 at meta-position exerts only -I effect, hence increases the acidity.
- I effect order :- NO2 > - OCH3 > - Cl.
- CH3 has +I effect. So, order is (a).
(iii) (d): Phenol (Carbolic acid) is a weaker acid than carbonic acid (H2CO3) and does not liberate CO2 on treatment with aqueous sodium bicarbonate solution.
(iv) (c): - NO2 exhibits both -I and -R influence to stabilise the corresponding phenoxide. In ortho derivative, intermolecular H-bonding lowers the acidity. -
(ii)
(iii) (a): When mixed ethers are used, the formation of alkyl iodide depends on the nature of alkyl groups. Methyl iodide is formed when one group is methyl and the other a primary or secondary alkyl group. Here reaction follows SN2 mechanism and because of the steric effect of the larger group, I- attacks the smaller (Me) group.
When the substrate is a methyl t-alkylether, the products are t-RI and MeOH. Here, reaction follows SN1 mechanism and formation of products is controlled by the stability of carbocation. Since, carbocation stability order is : 3° > 2° > 1° > CH3, therefore alkyl halide is always derived from tert-alkyl group.
(iv) (a): Diphenyl ethers are not cleaved by HBr or HI. -
(i) (c):
It has 9 C-atoms.
(iii) (b): Sodium phenoxide when heated with CO2 at 400 K under a pressure of 4-7 atm followed by acidification gives 2-hydroxybenzoic acid (salicylic acid) as the main product along with a small amount of 4-hydroxybenzoic acid. This reaction is called Kolbe's reaction.
Salol is used as an intestinal antiseptic. -
(i) (b): It is an electrophilic substitution reaction.
(ii) (c)
(iv) (b): It is known as Reimer-Tiemann reaction.
-
(i) (b): The order of dehydration of alcohols is 3° > 2° > 1°.
(ii) (b): The dehydration of alcohol is an example of elimination reaction.
(iii) (b): Dehydration of CH30H will give methylene (a carbene) which is unstable.
\(\mathrm{CH}_{3} \mathrm{OH} \stackrel{\mathrm{H}_{2} \mathrm{SO}_{4}}{\longrightarrow}: \mathrm{CH}_{2}+\mathrm{H}_{2} \mathrm{O}\)
(iv) (a):
-
(i) (a): Williamson's synthesis occurs by SN2 mechanism and primary alkyl halides are most reactive in SN2 reactions.
(ii) (c): Sodium methoxide is a strong nucleophile. In presence of a strong base, i.e., sodium methoxide, t-butyl bromide undergoes dehyrohalogenation to form isobutylene.
(iii) (d): Since secondary and tertiary alkyl halides prefer to undergo elimination rather than substitution, therefore, even symmetrical ethers containing secondary and tertiary alkyl groups cannot be prepared in good yields by Williamson synthesis.
(iv) (b): Depending upon whether the alkyl halide and the alkoxide ion carry the same or different alkyl groups, both symmetrical and unsymmetrical ethers can be prepared by Williamsons synthesis. -
(i) (b): Due to the presence of intermolecular H-bonding in alcohols, they have higher boiling points than isomeric ethers.
(ii) (c): Like alcohols, phenols also form H-bond with water. But the solubility of phenols in water is much lower than that of alcohols because of the larger non-polar hydrocarbon part (benzene ring) present in their molecules.
(iii) (d): Boiling point increases with increase in molecular mass so, 1-butanol has higher boiling point than 1-propanol. Ethers do not form hydrogen bonds thus, they have lower boiling points than the corresponding alcohols.
Due to weak dipole-dipole interactions, the boiling points of lower ethers are only slightly higher than those of the n-alkanes having comparable molecular masses. Thus, the increasing order of boiling points is n-butane < ethoxyethane <1-propanol < 1-butanol.
(iv) (b): The solubility of lower ethers in water is due to the formation of H-bonds between water and ether molecules. As the molecular mass increases, the solubility of ethers in water decreases due to corresponding increase in the hydrocarbon portion of the molecule. -
(i) (c): Both methanol and ethanol are 1° alcohols and hence, cannot be distinguished by Lucas' reagent.
(ii) (d): Tertiary alcohols immediately react with Lucas' reagent.
(iii) (d): The order of reactivity of alcohols towards Lucas' reagent is 3° alcohol > 2° alcohol > 1° alcohol. 1° alcohols do not react with Lucas' reagent at room temperature. It requires high temperature. The benzyl and allyl alcohols react as rapidly as 3° alcohols with Lucas' reagent because their cations are resonance stabilised.
(iv) (b) : When Lucas' reagent (conc. HCl + ZnCI2) is added to 2-propanol (2° alcohol) turibitity appears within five minutes whereas with 1-propanol no turbidity appears and solution remains dear at room temperature. -
(a) 2-methyl butan-2-ol
(b) Tertiary butyl alcohol will undergo dehydration faster because 3° carbocation is more stable.
(c)
(d)
(e) It is due to resonance in phenol, there is double bond character in C-O bond, therefore shorter than single bond in methanol.
(f)
(g) It is due to repulsive interaction between two lone pair of electrons in alcohol,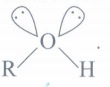
-
(a)
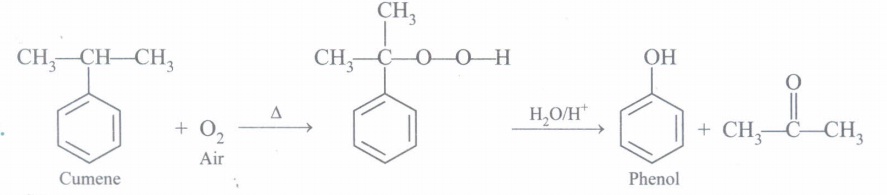
Acetone is obtained which is useful by product.
(b)
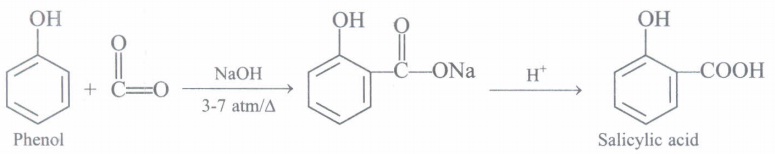
(c)
(d) Add neutral FeCI 3 . Phenol gives violet colour where as benzyl alcohol does not.
(e) It is due to oxidation. -
(a) It is due to inter molecular H-bonding.
(b) It is because it has spherical shape, least surface area, less van der Waals' forces of attraction, hence lower boiling point than n-butyl alcohol.
(c) Boiling point increases with increase in carbon chain
(d) Solubility of alcohol decreases with increase in molar mass.
(e) CH3OH is most acidic because \(\mathrm{CH}_{3} \mathrm{O}^{\ominus}\) is most stable among \(\mathrm{RO}^{\ominus}\) . -
(a) Picric acid
(b) Lower the pKa, more will be acidic character.
(c) It is because -CH3 groups are electron releasing, destabilise phenoxide ion.
(d) -OCH3 has more -I effect than -NH2 because oxygen is more electronegative than nitrogen.
(e) It is because in a-fluoro phenol, there is strong intramolecular H-bonding.






































