Class 12th Chemsitry - Coordination Compounds Case Study Questions and Answers 2022 - 2023
By QB365 on 08 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 12 Chemsitry Subject - Coordination Compounds, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Coordination Compounds Case Study Questions With Answer Key
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Read the passage given below and answer the following questions:
The molecular compounds which are formed from the combination of two or more simple stable compounds and retain their identity in the solid as well as in the dissolved state are called coordination compounds. Their properties are completely different from the constituents. In coordination compounds, the central metal atom or ion is linked to a number of ions or neutral molecules, called ligands, by coordinate bonds. For example, Dimethyl glyoxime (dmg) is a bidendate ligand chelating large amounts of metals. When dimethyl glyoxime is added to alcoholic solution of NiCl2 and ammonium hydroxide is slowly added to it, a rosy red precipitate of a complex is formed.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The structure of the complex is
(ii) Oxidation number of Ni in the given complex is(a) +3 (b) +1 (c) +2 (d) zero. (iii) Which of the following is true about this complex?
(a) It is paramagnetic, containing 2 unpaired electrons. (b) It is paramagnetic, containing 1 unpaired electron. (c) It is paramagnetic, containing 4 unpaired electrons. (d) It is diamagnetic with no unpaired electron (iv) Which one will give test for Fe3+ ions in the solution?
(a) [Fe(CN)6]3- (b) [Fe(CN)6]2- (c) (NH4)2SO4· FeSO4· 6H2O (d) Fe2(SO4)3 (a) -
Read the passage given below and answer the following questions:
Coordination compounds are formulated and named according to the IUPAC system.
Few rules for naming coordination compounds are:
(I) In ionic complex, the cation is named first and then the anion.
(II) In the coordination entity, the ligands are named first and then the central metal ion.
(III) When more than one type of ligands are present, they are named in alphabetical order of preference without any consideration of charge.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The IUPAC name of the complex \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{3} \mathrm{Br}\left(\mathrm{NO}_{2}\right) \mathrm{Cl}\right] \mathrm{Cl}\) is(a) triamminechlorobromonitroplatinum (IV) chloride (b) triamminebromonitrochloroplatinum (IV) chloride (c) triamminebromidochloridonitroplatinum (IV) chloride (d) triamminenitrochlorobromoplatinum (IV) chloride (ii) The lUPAC name of [Ni(CO)4] is
(a) tetracarbonylnickel (II) (b) tetracarbonylnickel (0) (c) tetracarbonylnickelate (II) (d) tetracarbonylnickelate (0). (iii) As per IUPAC nomenclature, the name of the complex \(\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\left(\mathrm{NH}_{3}\right)_{2}\right] \mathrm{Cl}_{3}\) is
(a) tetraaquadiamminecobalt (II) chloride (b) tetraaquadiamminecobalt (III) chloride (c) diamminetetraaquacobalt (II) chloride (d) diamminetetraaquacobalt (III) chloride (iv) Which of the following represents correct formula of dichloridobis( ethane-1, 2-diamine ) cobalt (III) ion?
(a) [CoCl2(en)]2+ (b) [Co(ONO)(NH3)5]SO4 (c) [Co(NO2)(NH3)4] (SO4)2 (d) [Co(NO)(NH3)4] (SO4)2 (a) -
Read the passage given below and answer the following questions:
Iron forms many complexes in its +2 and +3 oxidation states such as \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}(A) ;\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}(B)\) \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}(C) ;\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}(D)\) ,etc., They exhibit, different magnetic properties and undergo different hybridisation of iron.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Which of the following statements is correct?(a) (B) is paramagnetic while (C) is diamagnetic. (b) Both (B) and (D) are outer orbital complexes. (c) Both (A) and (C) are paramagnetic. (d) (A) is outer orbital complex and (C) is inner orbital complex. (ii) The complex having maximum magnetic moment is
(a) (A) (b) (B) (c) (C) (d) (D) (iii) The spin only magnetic moment of complexes (A), (B), (C) and (D) are respectively (in BM)
(a) \(2 \sqrt{6}, 0, \sqrt{35}, \sqrt{3}\) (b) \(0,2 \sqrt{6}, \sqrt{35}, \sqrt{3}\) (c) \(\sqrt{15}, 2 \sqrt{6}, \sqrt{3}, 0\) (d) \(\sqrt{3}, \sqrt{8}, 0, \sqrt{15}\) (iv) Which of the given complexes are outer orbital complexes?
(a) (A) and (B) only (b) (B) and (C) only (c) (A) and (C) only (d) (B) and (D) only (a) -
Read the passage given below and answer the following questions:
To explain bonding in coordination compounds various theories were proposed. One of the important theory was valence bond theory. According to that, the central metal ion in the complex makes available a number of empty orbitals for the formation ofcoordination bonds with suitable ligands. The appropriate atomic orbitals of the metal hybridise to give a set of equivalent orbitals of definite geometry. The d-orbitals involved in the hybridisation may be either inner d-orbitals i.e., (n - 1) d or outer d-orbitals i.e., nd. For example, CO3+ forms both inner orbital and outer orbital complexes, with ammonia it forms [Co(NH3)6]3+ and with fluorine it forms [CoF6]3- complex ion.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Which of the following is not true for [CoF6]3- ?(a) It is paramagnetic. (b) It has coordination number of 6. (c) It is outer orbital complex. (d) It involves d2sp3 hybridisation. Which of the following is true for [Co(NH3)6]3+ ?
(a) It is an octahedral, dimagnetic and outer orbital complex. (b) It is an octahedral, paramagnetic and outer orbital complex. (c) It is an octahedral, paramagnetic and inner orbital complex. (d) It is an octahedral, dimagnetic and inner orbital complex. (iii) The paramagnetism of [CoF6]3- is due to
(a) 3 electrons (b) 4 electrons (c) 2 electrons (d) 2 electrons (iv) Which of the following is an inner orbital or low spin complex?
(a) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}\) (b)\(\left[\mathrm{FeF}_{6}\right]^{3-}\) (c) \(\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}\) (d) \(\left[\mathrm{NiCl}_{4}\right]^{2-}\) (a) -
Read the passage given below and answer the following questions:
Valence bond theory considers the bonding between the metal ion and the ligands as purely covalent. On the other hand, crystal field theory considers the metal-ligand bond to be ionic arising from electrostatic interaction between the metal ion and the ligands. In coordination compounds, the interaction between the ligand and the metal ion causes the five d-orbitals to split-up. This is called crystal field splitting and the energy difference between the two sets of energy level is called crystal field splitting energy. The crystal field splitting energy (Δo) depends upon the nature of the ligand. The actual configuration of complexes is divided by the relative values of Δo and P (pairing energy)
If Δo < P, then complex will be high spin.
If Δo > P, then complex will be low spin.
The following questions are multiple choice questions. Choose the most appropriate answer :
(i) Which of the following ligand has lowest Δo value?(a) CN- (b) CO (c) F- (d) NH3 (ii) The crystal field splitting energy for octahedral (Δo) and tetrahedral (Δt) complex is related as
(a) \( \Delta_{t}=\frac{1}{2} \Delta_{o}\) (b) \(\Delta_{t}=\frac{4}{9} \Delta_{o}\) (c) \(\Delta_{t}=\frac{3}{5} \Delta_{o}\) (d) \(\Delta_{t}=\frac{2}{5} \Delta_{o}\) (iii) On the basis of crystal field theory, the electronic configuration of d4 in two situations: (i) Δo > P and (ii) Δo< P are
(i) (ii) (a) \(t_{2 g}^{4} e_{g}^{0}\) \(t_{2 g}^{3} e_{g}^{1}\) (b) \(t_{2 g}^{3} e_{g}^{1}\) \(t_{2 g}^{4} e_{g}^{0}\) (c) \(t_{2 g}^{3} e_{g}^{1}\) \(t_{2 g}^{3} e_{g}^{1}\) (d) \(t_{2 g}^{4} e_{g}^{0}\)
\(t_{2 g}^{4} e_{g}^{0}\) (iv) Using crystal field theory, calculate magnetic moment of central metal ion of [FeF6]4-.
(a) 1.79 B.M. (b) 2.83 B.M. (c) 3.85 B.M. (d) 4.9 B.M. (a) -
Read the passage given below and answer the following questions:
Metal carbonyl is an example of coordination compounds in which carbon monoxide (CO) acts as ligand. These are also called homoleptic carbonyls. These compounds contain both \(\sigma\) and \(\pi\) character. Some carbonyls have metal-metal bonds. The reactivity of metal carbonyls is due to (i) the metal centre and (ii) the CO ligands. CO is capable of accepting an appreciable amount of electron density from the metal atom into their empty \(\pi\) or \(\pi\)* orbitals. These types ofligands are called \(\pi\)-accepter or \(\pi\)-acid ligands. These interactions increases the Δo value.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) What is the oxidation state of metal in [ Mn2(CO)10] ?(a) +1 (b) -1 (c) +2 (d) 0 (ii) Among the following metal carbonyls, the C - O bond order is lowest in
(a) \(\left[\mathrm{Mn}(\mathrm{CO})_{6}\right]^{+}\) (b) \(\left[\mathrm{Fe}(\mathrm{CO})_{5}\right]\) (c) \(\left[\mathrm{Cr}(\mathrm{CO})_{6}\right]\) (d) \(\left[\mathrm{V}(\mathrm{CO})_{6}\right]^{-}\) (iii) The oxidation state of cobalt in K [CO(CO)4] is
(a) +1 (b) +3 (c) -1 (d) 0 (iv) Structure of decacarbonyl manganese is
(a) trigonal bipyramidial (b) octahedral (c) tetrahedral (d) square pyramidal. (a) -
Read the passage given below and answer the following questions:
Werner, a Swiss chemist in 1892 prepared and characterised a large number of coordination compounds and studied their physical and chemical behaviour. He proposed that, in coordination compounds, metals possess two types of valencies, viz. primary; valencies, which are normally ionisable and secondary valencies which are non-ionisable. In a series of compounds of cobalt (III) chloride with ammonia, it was found that some of the chloride ions could be precipitated as AgCI on adding excess of AgNO3 solution in cold, but some remained in solution. The number of ions furnished by a complex in a solution can be determined by precipitation reactions. The measurement of molar conductance of solutions of coordination compounds helps to estimate the number of ions furnished by the compound in solution.
In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Assertion: The complex [Co(NH3)3CI3] does not give precipitate with silver nitrate solution.
Reason: The given complex is non-ionisable.
(ii) Assertion: The complex [Co(NH3)4Cl2]Cl gives precipitate corresponding to 2 mol of AgCl with AgNO3 solution.
Reason: It ionises as [Co(NH3)4Cl2]+ + Cl-
(iii) Assertion: 1 mol of [CrCl2CH2O)4]Cl· 2H2O will give 1mol of AgCl on treating with AgNO3.
Reason: Cl- ions satisfying secondary valanceis will not be precipitated.
(iv) Assertion: CoCl3·3NH3 is not conducting while CoCl3·5NH3 is conducting.
Reason: The complex of CoCl3·3NH3 is [ CoCl3(3NH3)3] while that of CoCl3·5NH3 is [CoCl3(3NH3)5]Cl2.(a) -
Read the passage given below and answer the following questions:
Arrangement of ligands in order of their ability to cause splitting Δ is called spectrochemical series. Ligands which cause large splitting (large Δ ) are called strong field ligands while those which cause small splitting (small Δ) are called weak field ligands. When strong field ligands approach metal atomlion, the value of Δo is large, so that electrons are forced to get paired up in lower energy t2g orbitals. Hence, a low-spin complex is resulted from strong field ligand. When weak field ligands approach metal atom/ion, the value of Δo is small, so that electrons enter high energy eg orbitals rather than pairing in low energy t2g orbitals. Hence, a high-spin complex is resulted from weak field ligands. Strong field ligands have tendency to form inner orbital complexes by forcing the electrons to pair up. Whereas weak field ligands have tendency to form outer orbital complex because inner electrons generally do not pair up.
In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: In tetrahedral coordination entity formation, the d orbital splitting is inverted and is smaller as compared to the octahedral field splitting.
Reason: Spectrochemical series is based on the absorption of light by complexes with different ligands.
(ii) Assertion: F- ion is a weak field ligand and forms outer orbital complex.
Reason: F- ion cannot force the electrons of dz2 and dx2- y2 orbitals of the inner shell to occupy dxy, dyz and dzx orbitals of the same shell.
(iii) Assertion: The crystal field model is successful in explaining the formation, structures, colour and magnetic properties of coordination compounds.
Reason: In spectrochemical series, ligands are arranged in a series of increasing field strength.
(iv) Assertion: NF3 is a weaker ligand than N(CH3)3.
Reason: NF3 ionizes to give F- ions in aqueous solution.(a) -
Read the passage given below and answer the following questions:
Ligands are atoms or ions which can donate electrons to the central atoms. Ligands can be monodentate, bidentate or polydentate as well. Few ligands can coordinate with the central atom through more than one site, these are called ambidentate ligands. When a di- or polydentate ligand uses its two or more donor atoms to bind a single metal ion, it is said to be a chelating ligand.
In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Glycinate ion is an example of mono dentate ligand.
Reason: Glycinate contains Nand O as donor atoms
(ii) Assertion: Oxalate ion is a bidentate ligand.
Reason: Oxalate ion has two donor atoms
(iii) Assertion: A chelating ligand must possess two or more lone pairs at such a distance that it may form suitable strain free 5 and 6 membered rings with the metal ion.
Reason: H2N- NH2 is a chelating ligand.
(iv) Assertion: In Zeise's salt coordination number of Pt is five.
Reason: Ethene is a monodentate ligand.(a) -
Read the passage given below and answer the following questions:
For understanding the structure and bonding in transition metal complexes, the magnetic properties are very helpful. Low spin complexes are generally diamagnetic because of pairing of electrons, whereas high spin complexes are usually paramagnetic because of presence of unpaired electrons. Larger the number of unpaired electrons, stronger will be the paramagnetism. However magnetic behaviour of a complex can be confirmed from magnetic moment measurement. Magnetic moment \(\mu=\sqrt{n(n+2)} \text { B.M. }\)where n = number of unpaired electrons. Greater the number of unpaired electrons, more will be the magnetic moment.
In these questions ( i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Both [Cr(H2O)6]2+ and [FeH2O)6]2+ have same magnetic moment.
Reason: Number of unpaired electrons in Cr2+ and Fe2+ are same.
(ii) Assertion: [Fe(H2O)5NO]SO4 is paramagnetic.
Reason: The Fe in [Fe(H2O)5NO]SO4 has three unpaired electrons.
(iii) Assertion: [Co(en)3]3+ is paramagnetic.
Reason: It is an inner orbital complex.
(iv) Assertion: [Ni(CN)4]2- is diamagnetic complex.
Reason: It involves dsl hybridisation and there is no unpaired electron.(a) -
Complex compounds play an important role in our daily life. Werner's theory of complex compounds says every metal atom or ion has primary valency (oxidation state) which is satisfied by -vely charged ions, ionisable where secondary valency (coordination number) is non-ionisable, satisfied by Iigands (+ve, -ve, neutral) but having lone pair. Primary valency is non-directional, secondary valency is directional. Complex compounds are name according to IUPAC system. Valence bond theory helps in determining shapes of complexes based on hybridisation, magnetic properties, outer or inner orbital complex. Complex show ionisation, linkage, solvate and coordination isomerism also called structural isomerism. Some of them also show stereoisomerism i.e. geometrical and optical isomerism. Ambidentate ligand are essential to show linkage isomerism. Polydentate Iigands form more stable complexes then unidentate Iigands. There are called ehelating agents. EDTA is used to treat lead poisoning, cis-platin as anticancer agents. Vitamin B12 is complex of cobalt. Haemoglobin, oxygen carrier is complex of Fe2+ and chlorophyll essential for photosynthesis is complex of Mg2+.
(a) What is the oxidation state of Ni in [Ni(CO)4]?
(b) One mole of CrCI3 . 6H2O reacts with excess of AgO3 to yield 2 mole of AgCI. Write formula of complex. Write IUPAC name also.
(c) Out Cis - [Pt(en)2 CI2 ]2+ and trans (Ptren), CI2 )2+ which one shows optical isomerism?
(d) Name the hexadentate ligand used for treatment of lead poisoning.
(e) What is hybridisation of [CoF6]3- [Co = 27] Give its shape and magnetic properties.
(f) Out [Fe(CO)5], [Fe(C2 O4 )3]3-, [Fe(H2O6)3+, [Fe(CN)6]3-, which is most stable?
(g) What type of isomerism is shown by [Cr(H2O)6] CI3 and [Cr(H2O)5 CI] CI2 . H2O?(a) -
Transition metals form complex compounds which playa very important role in our daily life. Complexes are also formed by other groups elements e.g. Chlorophyll is coordination compound of Mg. Organometallic compounds like Grignard reagent is most useful in organic chemistry. Complexes are used in medicines, analytical chemistry, qualitative analysis, electroplating, biological processes. Stability of complexes depends upon charge on central metal ion, strength of ligand. Counter ions outside the coordination entity are ionisable but inside the coordination sphere are not ionisable.
(a) Name a complex used as anticancer agent?
(b) What is coordination number of Co in [Co(en)3]3+ and why?
(c) Name a complex used in Gold plating
(d) Name a complex used for determining hardness of water. What is its denticity?
(e) How is undecomposed AgBr removed from photographic film? Write the reaction involved.(a) -
Observe the table related to stability constant of some complex compounds. Answer the questions based on the table and related concepts.
Stability Constants of Some ComplexesComplex Stability Constant (b) [Cu(NH3 )4]2+ 4.5 x 1011 [Cu(CN)4]2- 2.0 x 1027 Ag(NH3 )2]+ 1.6 x 107 [Co(NH3 )6]3+ 5.0 x 1033 \(\left[\mathrm{Ag}(\mathrm{CN})_{2}\right]^{\ominus}\) 5.4 x 1018 [Ni(NH3 )6]2+ 6.1 x 1018 [Ni( en)3]2+ 4.6 x 1018 [Fe(CN)6]3- 1.2 x 1031 [Fe(CN)6]4- 1.8 x 106 [Cd(NH3 )4]2+ 1.0 x 107 (a) Why stability constants of cyanides complexes higher than complexes with NH3?
(b) Why is (Cu(NH3 )4]2+ more stable than (Cd(NH3 )4]2+?
(c) Why is (Ni(en)3]2+ more stable than (Ni(NH3)6]2+?
(d) Why is (Fe(CN)6]3- more stable than IFe(CN)6]4-?
(e) What is chelate effect?(a) -
Observe the diagram of splitting of d-orbitals in octahedral field and answer the questions based on the diagrams and related studied concepts.
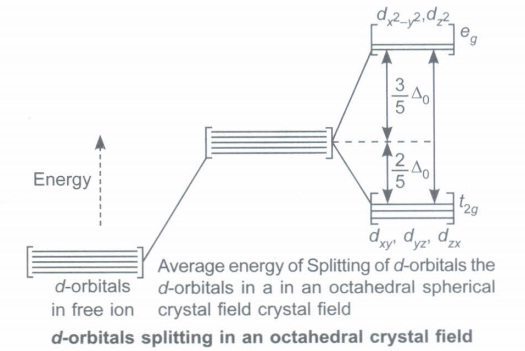
(a) What is crystal field splitting anergy?
(b) Why dx2-y2 ,dz2 have higher energy than dxy, dyz, dzx orbitals in octahedral crystal field?
(c) What is relationship between (CFSE) \(\triangle\)0 and strength of ligand?
(d) What is electronic configuration of d5 ion if \(\triangle\)0 < P?
(e) What is spectrochemical series?(a)
Case Study
*****************************************
Answers
Coordination Compounds Case Study Questions With Answer Key Answer Keys
-
(ii) (c)
(iii) (d) : It has no unpaired electrons hence, it is diamagnetic.
(iv) (d): (a) and (b) are coordination compounds hence cannot give free Fe2+ or, Fe3+ ions in solution. (c) and (d) represent simple compounds hence are free to give ions in solution, but only Fe2(SO4)3 contains Fe3+ ions.(NH4)2SO4・FeSO4・ 6H2O contains Fe2+ ions not Fe3+ ions. -
(i) (c): Ligands are named in alphabetical order irrespective of their charge.
(ii) (b)
(iii) (d)
(iv) (d) -
(i) (c): (A) : sp3d2 hybridisation (outer orbital)
No. of unpaired electrons = 4
(B) : d2sp3 hybridisation (inner orbital)
No. of unpaired electrons = 0
(C) : sp3 d2 hybridisation (outer orbital)
No. of unpaired electrons = 5
(D) : d2sp3 hybridisation (inner orbital)
No. of unpaired electron = 1
(ii) (c): It has 5 unpaired electrons.
(iii) (a): Magnetic moments of (A), (B), (C) and (D) are respectively.
\(\sqrt{4(4+2)}, 0, \sqrt{5(5+2)}, \sqrt{1(1+2)}\)
(iv) (c) -
(i) (d): It involves sp3 d2 hybridisation and not d2sp3.
(ii) (d) : [Co(NH3)6]3+ is d2sp3 hybridised with all electrons paired hence, it is diamagnetic and inner
orbital complex.
(iv) (c): Inner orbital complexes are formed with strong ligands as they force electrons to pair up and hence the complex will be either diamagnetic or will have less number of unpaired electrons. -
(i) (c): Spectrochemical series:
\((\mathrm{I}^{-}<\mathrm{Br}^{-}<\mathrm{SCN}^{-}<\mathrm{Cl}^{-}<\mathrm{S}^{2-}<\mathrm{F}^{-}<\mathrm{OH}^{-}<\mathrm{C}_{2} \mathrm{O}_{4}^{2-}<\mathrm{O}^{2-}< \mathrm{H}_{2} \mathrm{O}<\mathrm{NCS}^{-}<\mathrm{EDTA}^{4-}<\mathrm{NH}_{3}\)(ii) (b)
(iii) (a) : When Δo > P, the electrons paired up in the t2g level rather than going to the eg level, so
when Δo> P : \(t_{2 g}^{4} e_{g}^{0}\)
and Δo < P : \(t_{2 g}^{3} e_{g}^{1}\)
(iv) (d): Fe2+: 3d6 ⇒\(t_{2 g}^{4} e_{g}^{2}\)
(Since, F- is a weak field ligand)
Hence four unpaired electrons are present.
Magnetic moment (μ)\(=\sqrt{n(n+2)}=\sqrt{4(4+2)}=4.9 \mathrm{~B} \cdot \mathrm{M}\) -
(i) (d): Oxidation state of Mn in [Mn2(CO)10] is zero.
(ii) (d): In \(\left[\mathrm{V}(\mathrm{CO})_{6}\right]^{-}\), the anionic carbonyl complex can delocalise more electron density to antibonding \(\pi\)-orbital (d\(\pi\)-p\(\pi\) back bonding) of CO and thus lowers the bond order.
(iii) (c): K[CO(CO)4]
+ 1 + (x) + 4(0) = 0 or x = -1
(iv) (d): Mn2(CO)10 is made up of two square pyramidal Mn(CO)5 units joined by Mn - Mn bond. -
(i) (a)
(ii) (d): [Co(NH3)4Cl2]Cl + AgNO3➝[Co(NH3)4Cl2]+ + AgCl ↓
Thus it gives precipitate of 1 mol of AgCl.
(iii) (a) : The Cl ions outside the coordination sphere can only be precipitated.
(iv) (a) -
(i) (b)
(ii) (a)
(iii) (b)
(iv) (c): Due to high electronegativity of F- atoms, the lone pair of N-atom in NF3 molecule cannot be ligated easily. Whereas in N(CH3)3' CH3 group is a electron releasing group, thus lone pair of N-atom in N(CH3)3 molecule can be ligated easily.
Except, nitrogen fluoride, all other halides hydrolyse in water. -
(i) (d): Glycinate ion is an example of bidentate ligand. It contains Nand O as donor atoms.
(ii) (a)
(iii) (c) : H2N - NH2 does not act as chelating ligand.
The coordination by hydrazine leads to a three member highly unstable strained ring and thus it does not act as chelating agent.
(iv) (d): In Zeises salt, coordination no. of Pt is 4. Ethylene is a mono dentate ligand. -
(i) (a): Spin only magnetic moment, \(\mu=\sqrt{n(n+2)} \text { B.M. }\) where n = number of unpaired electrons.
As the number of unpaired electrons in Cr2+ ([Ar ]3d4) and Fe2+([Ar]3d6) are same, hence [Cr(H2O)6]2+ and [Fe(H2O)6]2+ will have same magnetic moment.
(ii) (a): Fe+ : [Ar] 3d6 4s1
When the weak field ligand H2O and strong field ligand NO+ attack, the configuration changes as follows: Fe+ : [Ar] 3d7 4s°
ஃ Fe+ has 3 unpaired electrons.
In presence of strong ethylenediamine ligand the electrons get paired.
Thus inner orbital complex with no unpaired electrons.
(iv) (a) -
(a) Zero
(b) [Cr(H2O)5Cl]CI2 . H2O, pentaaqua chlorido chromium (III) chloride.
(c) Cis - [Pt(en)2 Cl2]2+ shows optical isomerism.
(d) EDTA4- (ethylene diamine tetra acetate)
(e) Sp3 d2, octahedral, paramagnetic. It is outer orbital complex.
(f) [Fe(CO)5] is most stable because CO is strongest ligand.
(g) Solvate isomerism. -
(a) Cis-platin
(b) Coordination number of Co is 6 because 'en' (ethane 1, 2-diammine) is bidentate ligand.
(c) \(\left[\mathrm{An}(\mathrm{CN})_{2}\right]^{\ominus}\) Dicyanido aurate(1)
(d) EDTA, it is hexadentate ligand.
(e) It is done by reacting with sodium thio sulphate
AgBr + 2Na2S2O3 \(\rightarrow\) Na3[Ag(SP3)2] + NaBr
Sodium dithio sulphato argentate (I) -
(a) It is because CN- is stronger ligand than NH3 .
(b) t is because Cu 2 + is smaller in size than Cd2 +, form more stable complex.
(c) It is because 'en' is bidentate ligand, forms more stable complex [Ni(en)3]2+ than [Ni(NH3 )6]2+.
(d) It is because Fe3 + is small and has higher positive charge than Fe2 +.
(e) The stabilisation due to chelation by bidentate and polydentate ligands is called chelation. -
(a) The energy difference between the two sets of d-orbitals is called crystal field splitting energy denoted by \(\triangle\)0 .
(b) The orbitals dx2y2, dz2 lying in the direction of ligands, will experience greater repulsion and their energies will be raised relative to their positions in symmetrical field as compared to orbitals dxy, dyz, dzx lying in between the axis (at angle of 45°), lying away from the approach of ligand.
(c) Greater the (CFSE) \(\triangle\)0 more will be strength of ligand.
(d) \(t_{2 g}^{3} e g^{2}\)
(e) The series in which ligands are arranged in increasing order of magnitude of crystal field splitting energy (\(\triangle\)0 ) is called spectrochemical series.






































