Class 12th Chemsitry - Haloalkanes and Haloarenes Case Study Questions and Answers 2022 - 2023
By QB365 on 08 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 12 Chemsitry Subject - Haloalkanes and Haloarenes, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Haloalkanes and Haloarenes Case Study Questions With Answer Key
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Read the passage given below and answer the following questions:
A primary alkyl halide (A) C4H9Br reacted with alcoholic KOH to give compound (B). Compound (B) is reacted with HBr to give compound (C) which is an isomer of (A). When (A) reacted with sodium metal, it gave a compound (D) C8H18 that is different than the compound obtained when n-butyl bromide reacted with sodium metal
The following questions are multiple choice questions. Choose the most ap appropriate answer:
(i) Compound (A) is(a) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Br}\) (b) (c) (d) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Br}\) (ii) Which type of isomerism is present in compound (A) and (C)?
(a) Positional (b) Functional (c) Chain (d) Both (a) and (c) (iii) IUPAC name of compound (D) is
(a) n-octane (b) 2,5-dimethylhexane (c) 2-methylheptane (d) 3,4-dimethyl hexane. (iv) When compoound (C) is treated with alc. KOH and then treated with HBr in presence of peroxide, the compound obtained is
a) (b) (c) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Br}\) (d) (a) -
Read the passage given below and answer the following questions:
Nucleophilic substitution reactions are of two types; substitution nucleophilic bimolecular (SN2) and substitution nucleophilic unimolecular (SN1) depending on molecules taking part in determining the rate of reaction. Reactivity of alkyl halide towards SN1 and SN2 reactions depends on various factors such as steric hindrance, stability of intermediate or transition state and polarity of solvent. SN2 reaction mechanism is favoured mostly by primary alkyl halide then secondary and then tertiary. This order is reversed in case of SN1 reactions.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Which of the following is most reactive towards nucleophilic substitution reaction?(a) \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{Cl}\) (b) \(\mathrm{CH}_{2}=\mathrm{CHCl}\) (c) \(\mathrm{ClCH}_{2} \mathrm{CH}=\mathrm{CH}_{2}\) (d) \(\mathrm{CH}_{3} \mathrm{CH}=\mathrm{CHCl}\) (ii) Isopropyl chloride undergoes hydrolysis by
(a) SN1mechanism (b) SN2mechanism (c) SN1 and SN2mechanism (d) neither SN1 and SN2mechanism (iii) The most reactive nucleophile among the following is
(a) CH3O- (b) C6H5O- (c) (CH3)2CHO- (d) (CH3)3CO- (iv) Tertiary alkyl halides are practically inert to substitution by SN2mechanism because of
(a) insolubility (b) instability (c) inductive effect (d) stearic hindrance. (a) -
Read the passage given below and a.9swer the following questions:
A chlorocompound (A) on reduction with Zn-Cu and ethanol gives the hydrocarbon (B) with five carbon atoms. When (A) is dissolved in dry ether and treated with sodium metal it gave 2,2,5,5 tetramethylhexane. The treatment of (A) with alcoholic KCN gives compound (C).
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The compound (A) is(a) 1-chloro- 2, 2-dimethylpropane (b) 1-chloro- 2, 2-dimethyl butane (c) 1-chloro-2-methyl butane (d) 2-chloro-2-methyl butane. (ii) The reaction of (C) with Na, C2H5OH gives
(a) (CH3)3C CH2CONH2 (b) (CH3)3C NH2 (c) (CH)3C CH2CH2NH2 (d) (CH3)2CHCH2NH2 (iii) The reaction of (C) with Na, C2H5OH is called
(a) Gilman reaction (b) Mendius reaction (c) Grooves process (d) Swart's reaction. (iv) Compound (B) is
(a) n-pentane (b) 2, 2-dimethylpropane (c) 2-methylbutane (d) none of these. (a) -
Read the passage given below and answer the following questions:
When haloalkanes with \(\beta\)-hydrogen atom are boiled with alcoholic solution of KOH, they undergo elimination of hydrogen halide resulting in the formation of alkenes. These reactions are called \(\beta\)-elimination reactions or dehydrohalogenation reactions. These reactions follow Saytzeff's rule. Substitution and elimination reactions often compete with each other. Mostly bases behave as nucleophiles and therefore can engage in substitution or elimination reactions depending upon the alkyl halide and the reaction conditions.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Among the following the most reactive towards alcoholic KOH is(a) \(\mathrm{CH}_{2}=\mathrm{CHBr}\)
(b) \(\mathrm{CH}_{3} \mathrm{COCH}_{2} \mathrm{CH}_{2} \mathrm{Br}\)
(c) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{Br}\)
(d) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Br}\)
(ii) The general reaction, \(R-X \stackrel{\text { aq. } \mathrm{OH}^{-}}{\longrightarrow} R \mathrm{OH}+X^{-}\) is expected to follow decreasing order of reactivity as in (t- Bu = tertiary Butyl group)
(a) t-BuI> t-BuBr > t-BuCI > t-BuF (b) t-BuF> t-BuCI > t-BuBr > t-BuI (c) t-Bu'Br> t-BuCI > t-BuI > t-BuF (d) t-BuF> t-BuCI > t-BuI > t-BuBr (iii) Reaction of t-butyl bromide with sodium methoxide produces
(a) sodium t-butoxide (b) t-butyl methyl ether (c) iso-butane (d) iso-butylene. (iv) In the elimination reactions, the reactivity of alkyl halides follows the sequence
(a) R - F > R - Cl > R - Br > R - I (b) R - I > R - Br > R - Cl > R - F (c) R - I > R - F > R - Br > R - Cl (d) R - F > R-I > R-Br > R-CI (a) -
Read the passage given below and answer the following questions:
Consider the given sequence of reactions:
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Identify w.(ii) When X reacts with CH3COCI in presence of anhy. AICI3, the reaction is known as
(a) Fittig reaction (b) Ullmann reaction (c) Wurtz-Fittig reaction (d) Friedel-Crafts acylation reaction. (iii) When X is treated Ni-Al / NaOH the product obtained is
(a) benzene (b) phenol (c) p-chlorophenol (d) triphenyl. (iv) Compound Z is
(a) phenol (b) p-chlorophenol (c) p-nitrophenol (d) nitrobenzene (a) -
Read the passage given below and answer the following questions:
Haloarenes are less reactive than haloalkanes. The low reactivity of haloarenes can be attributed to
(i) resonance effect
(ii) Sp2 hybridisation of C - X bond
(iii) polarity of C - X bond
(iv) instability of phenyl cation (formed by self-ionisation ofhaloarene)
(v) repulsion between the electron rich attacking nucleophiles and electron rich arenes.
Reactivity of haloarenes can be increased or decreased by the presence of certain groups at certain positions for example, nitro (-NO2) group at olp positions increases the reactivity of haloarenes towards nucleophilc substitution reactions.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Aryl halides are less reactive towards nucleophilic substitution reaction as compared to alkyl halides due to(a) the formation ofless stable carbonium ion (b) resonance stabilisation (c) larger carbon-halogen bond (d) inductive effect. (ii) Which of the following aryl halides is the most reactive towards nucleophilic substitution?
(iii) Which one of the following will react fastest with aqueous NaOH?
The reactivity of the compounds (i) MeBr, (ii) PhCH2Br, (iii) MeCI, (iv) p- MeOC6H4Br decreases as
(a) (i) > (ii) >(iii) > (iv) (b) (iv) > (ii) >(i) > (iii) (c) (iv) > (iii) >(i) > (ii) (d) (ii) > (i) > (iii) > (iv) (a) -
Read the passage given below and answer the following questions:
In haloalkanes, when a nucleophile stronger than the halide ion approaches the positively charged carbon atom of an alkyl halide, the halogen atom along with its bonding electron pair gets displaced and a new bond with the carbon and the nucleophile is formed. These reactions are called nucleophilic substitution reactions.
In these reactions the atom or group of atoms which loses its bond from carbon and takes on an additional pair of electrons is called leaving group. Halide ions are good leaving groups. Some important nucleophilic substitution reactions of haloalkanes with common nucleophiles are given in the table below.Reagent Nudeophile (Nu-) Substitution product
R-NuClass of main product 1. NaOH or KOH or moist Ag2O -OH ROH Alcohol 2. H2O H2O ROH Alcohol 3. NaI I- R-I Alkyl iodide 4. R'NH 2 \(R^{\prime} \ddot{\mathrm{N}} \mathrm{H}_{2}\) RNHR' Sec. amine 5. KCN \(\overline{\mathrm{C}} \equiv \mathrm{N}:\) RCN Nitrile (cyanide) 6. KNO2 O=N-O- R-O-N=O Alkyl nitrite In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason' is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Alkyl halides are hydrolysed to alcohols by moist silver oxide.
Reason: RCI is hydrolysed to ROH easily but reactions slow down on addition of KI.
(ii) Assertion : Alkyl halides form alkenes when heated above 300°C.
Reason: CH3CH2I reacts slowly with strong base as compared to CD3CH2I.
(iii) Assertion : RBr reacts with AgNO2 to give nitro alkane.
Reason: Silver nitrite (AgNO2) is an ionic compound, therefore the negative charge on nitrogen is the attacking site.
(iv) Assertion: The nucleophilic substitution of vinyl chloride is difficult than ethyl chloride.
Reason: Vinyl group is electron donating group.(a) -
Read the passage given below and answer the following questions:
When a chemical reaction involves bond cleavage or bond formation at an asymmetric carbon atom, three different products may be formed. For example, during the substitution of a group X by Y in the following reaction, the three possible products may be shown below:
(i) If B is the only product, the process is called retention of configuration because B has the same configuration as the starting reactant (A).
(ii) If C is the only product, the process is called inversion of configuration because C has the configuration opposite to the starting reactant (A).
(iii) If an equimolar mixture of B and C (i.e., a 50 : 50 mixture) is formed, then the process is called racemisation and the product is optically inactive because one isomer will rotate the light in the direction opposite to another.
In these questions ( i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correctstatements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: A reaction is said to be stereospecific if a particular stereoisomer of the reactant produces a specific stereoisomer of the product.
Reason: Bromination of cis-2-butene gives meso-2, 3-dibromobutane which is stereospecific
(ii) Assertion: Addition of Br2 to cis-but-2-ene is stereoselective.
Reason: SN2 reactions are stereospecific as well as stereoselective.
(iii) Assertion: Optically active 2-iodobutane on treatment with NaI in acetone undergoes recemization.
Reason: Repeated Walden inversions on the reactant and its product eventually gives a racemic mixture.
(iv) Assertion: SN2 reaction of an optically active alkyl halide with an aqueous solution of KOH always gives an alcohol with opposite sign of rotation.
Reason: SN2 reactions always proceed with inversion of configuration.(a) -
Read the passage given below and answer the following questions:
The order of reactivity towards SN1 reaction depends upon the stability of carbocation in the first step. Greater the stability of the carbocation, greater will be its ease of formation from alkyl halide and hence faster will be the rate of the reaction. As we know, 3° carbocation is most stable, therefore, the tert-alkyl that halides will undergo SN1 reaction very fast. For example, it has been observed that the reaction (CH3)3CBr with OH- ion to give 2-methyl-2-propanol is about 1 million times as fast as the corresponding reaction of the methyl bromide to give methanol.
The primary alkyl halides always react predominantly by SN2 mechanism. On the other hand, the tertiary alkyl halides react predominantly by SN1 mechanism. Secondary alkyl halides may react by either mechanism or by both the mechanisms without much preference depending upon the nature of the nucleophile and solvent.
In these questions ( i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Low concentration of nucleophile favours SN1mechanism.
Reason: 2° alkyl halides are less reactive than 1° towards SN1 reactions.
(ii) Assertion: Polar solvent slows down SN2reactions.
Reason: CH3-Br is less reactive than CH3Cl.
(iii) Assertion: Benzyl bromide when kept in acetone- water it produces benzyl alcohol.
Reason: The reaction follows SN2 mechanism.
(iv) Assertion: Rate of hydrolysis of methyl chloride to methanol is higher in DMF than in water.
Reason: Hydrolysis of methyl chloride follows second order kinetics.(a) -
Read the passage given below and answer the following questions:
The aryl halides are relatively less reactive towards nucleophilic substitution reactions as compared to alkyl halides. This low reactivity can be attributed to the following factors:
1. The C - X bond in halobenzene has a partial double bond character due to involvement of halogen electrons in resonance with benzene ring.
2. The C - X bond in aryl halides is less polar as compared to that in alkyl halides as sp2 hyridised carbon is more electronegative than sp3 hybridised carbon.
In these questions ( i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Primary benzylic halides are more reactive than primary alkyl halides towards SN1 reactions.
Reason: Reactivity depends upon the nature of the nucleophile and the solvent.
(ii) Assertion :is more reactive than
towards nucleophilic substitution reactions.
Reason: Tertiary alkyl halides react predominantly by SN1 mechanism.
(iii) Assertion: Chlorobenzene is more reactive than p-chloroanisole to nucleophilic substitution reactions.
Reason: Greater the stability of'carbanion, greater is its ease of formation and hence, more reactive is the aryl halide.
(iv) Assertion: Chlorobenzene is less reactive than benzene towards the electrophilic substitution reaction.
Reason: Resonance destabilises the carbo cation.(a) -
Haloalkanes are colourless (when pure), sweet smelling liquids. CH3Cl, CH3Br and C2H5CI and freons are gases. Boiling point increases with increase in molecular weight and increase in carbon chain and decreases with branching. They are insoluble in water due to inability to form H-bonds with water. Dipole moment increases with polarity, density increases with increase in molar mass. They are non-inflammable, therefore, CCl4 is used as fire extinguisher under the name pyrene. p-dichloro benzene has zero dipole moment, higher melting point than o-dichloro benzene due to symmetry, fits into crystal lattice readily. Haloalkanes undergo nucleophilic substitution reaction by SN2 mechanism, 1° > 2° > 3°, SN1 if carbocation formed is stable. They also undergo nucleophilic elimination reactions with alcoholic KOH. Haloarenes are less reactive than haloalkanes towards nucleophilic substitution due to resonance. Haloarenes undergo electrophilic substitution reaction like nitration, sulphonation, Friedel Crafts alkylation, acylation. Chloroform is used as solvent, Freon is used as refrigerant, dichloromethane is used as paint remover. Iodoform is used as antiseptic. DDT is insecticide but nonbiodegradable.
(a) Arrange R-F, R-Br, R-I, R-CI in increasing order of boiling point.
(b) A hydrocarbon' A' (C5H10) gives only one monochloro product on photo chlorination. Identity 'A'.
(c) Out of CH2 = CH-CH2CI and CH3CH2CH2Cl which one undergoes SN1 mechanism faster?
(d) Complete the following:
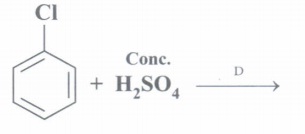
(e) Why is chloroform stored in dark coloured bottles?
(f) What is use of fully fluorinated organic compounds?
(g) Name the chlorine containing drug used in treatment of coronavirus and malaria.(a) -
The substitution reaction of alkyl halides occurs in SN1 or SN2 mechanism whatever mechanism alkyl halide follow for substitution reaction to occur, the polarity of the carbon-halogen bond is responsible for the substitution reaction. The rate of SN1 reactions are governed by the stability of carbocation where as for SN2 reactions steric factor is the deciding factor. If the starting material is a chiral compound, we may end up with an inverted product or racemic mixture depending upon the type of mechanism followed by alkyl halide. Cleavage of ethers with HI is also governed by steric factor and stability of carbocation which indicates that in organic chemistry, these two major factors help us in deciding the kind of product formed.
(a) Predict the stereochemistry of the product formed if optically active alkyl halide undergoes substitution reaction by SN1 mechanism.
(b) Name the instrument used for measuring the angle bl which the plane polarised light is rotated.
(c) Predict the major product formed when 2-bromopentane reacts with alcoholic KOH.
(d) Write the structure of the products formed when anisole is treated with ID.
(e) Write the structure of product formed when ethoxy benzene is treated with HI.(a) -
Observe the histogram related by comparison of boiling points of some alkyl halides and answer the questions that follow
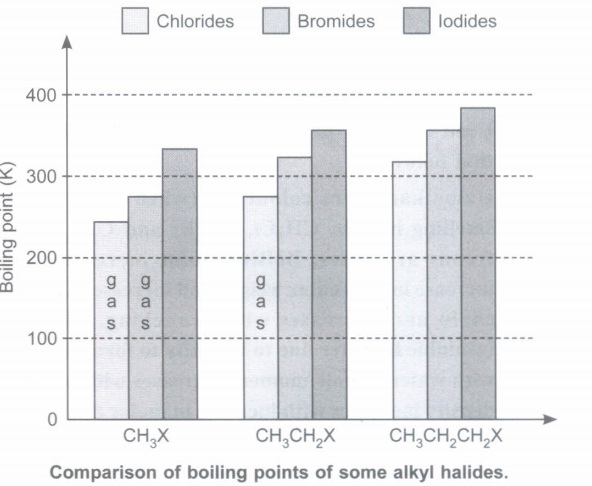
(a) Name the alkyl halides in gaseous state.
(b) Why do alkyl iodides have highest boiling points among alkyl halides?
(c) How does boiling point vary with increase in carbon chain?
(d) What is effect of branching on boiling point? Why?
(e) What happens to inflammability with increase in halogen atoms? Illustrate with example.(a) -
Observe the table in which physical data of Halo methane, polyhalogen derivative and haloarenes is given. Answer the questions based on them.
Table: Some Physical Data of Halomethanes (CH3-X)Bond C-X length Bond enthalpy Dipole moment 1 CH3F 139 452 1.847D 2 CH3Cl 178 351 1.860D 3 CH3Br 193 293 1.83D 4 CH3I 214 234 1.636D 5 CH2Cl2 - - 1.62D 6 CHCl3 - - 1.03D 7 CCl 4 - - Zero Dipole moment Melting point 8 p-dichloro benzene Zero 325K 9 o-dichloro benzene 2.54D 216K 10 m-dichloro benzene 1.72D 249K Boiling point 11 C6H5F 1.60D 358K 12 C6H5CI 1.69D 405K 13 C6H5Br 1.70D 429K 14 C6H5I 1.70D 462K (a) Why is dipole moment of CH3F less than CH3CI?
(b) Why is CH3I more reactive than CH3Cl?
(c) Why is melting point of p-dichlorobenzene higher than o-dichlorobenzene but boiling point of o-isomer is higher than p-isomer?
(d) Why does o-dichlorobenzene has higher dipole moment than m-dichlorobenzene?
(e) Why does C6H5I has higher dipole moment than C6H5F?(a)
Case Study
*****************************************
Answers
Haloalkanes and Haloarenes Case Study Questions With Answer Key Answer Keys
-
(i) (b): When compound (A) reacted with Na-metal, it gave a compound D(C8H18) which is different from the compound obtained when n-butyl bromide reacted with Na metal and hence the
compound (A) must be isobutyl bromide.
\(2 \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Br}+2 \mathrm{Na}\) \(\overset{Wurtz reaction}\rightarrow\) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}\)
-
(i) (c) : Allylic chlorides are most reactive.
(ii) (c): 20 - alkyl halides undergo hydrolysis by SN1 or SN2 mechanism.
(iii) (a): Smaller the size of the nucleophile (i.e., CH3O-), more reactive it is.
(iv) (d): Stearic hindrance due to bulky alkyl groups prevents the attack of the nucleophile in SN2 mechanism. -
(iii) (b)
-
(i) (d): In alkyl halides, polarity of C - Br bond increases with increase in chain length.
(ii) (a): The order of reactivity of alkyl halides: iodide > bromide > chloride (nature of the halogen atom)
tertiary> secondary> primary (type of halogen atom).
(iii) (d) : Iso-butylene is obtained.
(iv) (b): The order of bond dissociation energy: R - F > R - CI > R - Br > R - I. During dehydrohalogenation C - I bond breaks more easily than C - F bond. So reactivity order of halides R - I > R - Br > R - CI > R - F -
-
(i) (b)
(ii) (d): When in aryl halides the electron withdrawing groups are attached at ortho and para positions to the chlorine atom then the removal of chlorine atom as CI- ion becomes easy, therefore, 2,4,6- trinitro chlorobenzene is the most reactive among given aryl halides.
(d) : The order of reactivity follows the sequence: benzyl halides> alkyl halides> aryl halides. Out of chlorides and bromides, bromides are more reactive. Therefore, the correct order of reactivity is PhCH2Br(ii) > MeBr(i) > MeCI(iii) >p-MeOC6H4Br (iv). -
(i) (c) : KI reacts with RCI to form RI. This RI molecule now hydrolysed easily to give ROH because alkyl iodide are more reactive than alkyl chloride. Thus, reaction becomes faster on addition of KI.
(ii) (c): CH3CH2I reacts more rapidly with strong base in comparison to CD3CH2I. The elimination of HI (or DI) in presence of strong base shows E2 elimination. The rate determining step involves the breaking up of C - H (or C - D) bond. The C - D bond being stronger than C - H bond is difficult to break.
(iii) (c) : Silver nitrite is a covalent compound and the bond between Ag - O is covalent. Therefore, it does not have a negative charge on the oxygen atom. Hence, the nucleophillic attack occurs through the lone pair on nitrogen forming nitro alkanes (R - NO2).
(iv) (c): The carbon-halogen bond in vinyl halides has some double bond character and hence little difficult to break. -
(i) (c): Bromination of cis-2-butene give (±) 2,3-dibromobutane.
(ii) (b)
(iii) (a)
(iv) (a) -
(i) (c) : Ability to accommodate a positive charge determines the ease of heterolysis leading to SN1 mechanism. This ability to accommodate positive charge is more in the 20 alkyl halide since it has two alkyl groups as compared to one in 10 alkyl halide .
(ii) (c): CH3 - Br is more reactive than CH3 - Cl. The C - Br has bond dissociation energy of 293 kJ mol-1 while C - CI bond has its dissociation energy of 351 kJ mol-1. As the bond dissociation energy increases, the ease of breaking of C - X bond decreases and hence the reactivity of haloalkanes decreases.
(iv) (a) -
(i) (b): Primary benzylic halides show higher reactivity in SN1reactions than primary alkyl halides. This is due to the greater stabilisation of the benzylic carbocation intermediates by resonance.
(ii) (a)
(iii) (a) : On comparing the relative stabilities of carbanion of chlorobenzene and p-chloroanisole.
the electron donating group (OCH3) in anisole tends to intensify the negative charge relative to carbanion in chlorobenzene. Thus, p-chloroanisole is less reactive than chlorobenzene.
(iv) (c): Chlorobenzene is less reactive than benzene towards the electrophilic substitution reactions due to -I effect. -
(a) R-F < R-Cl < R-Br < R-I
(b)

(c) CH2 =CH-CH2Cl because \(\mathrm{CH}_{2}-\mathrm{CH}-\mathrm{CH}_{2}^{\oplus}\) is more stable due to resonance.
(d)
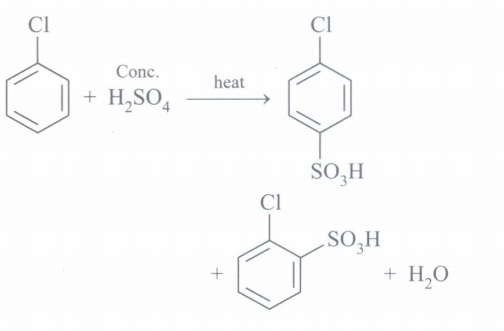
(e) It is done so as to prevent formation of COCl2 which is poisonous.
\(\mathrm{CHCl}_{3}+\frac{1}{2} \mathrm{O}_{2} \stackrel{\text { Sunlight }}{\longrightarrow} \mathrm{COCl}_{2}+\mathrm{HCl}\)
(f) They are used as blood substitute in surgery.
(g) Hydroxychloroquine -
(a) Racemic mixture will be formed.
(b) Polarimeter
(c) Pent-2-ene will be major product.
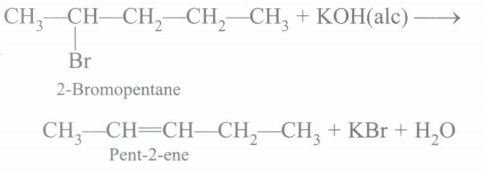
(d) Phenol and CH3I are formed
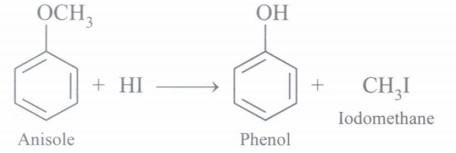
(e)
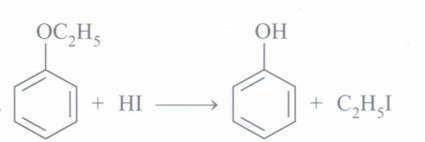
-
(a) CH3Cl (chloromethane), CH3Br (bromomethane), CH3CH2Cl (chloroethane)
(b) It is due to higher molar mass, bigger size, more van der Waals' forces of attraction.
(c) As the carbon chain increases, surface area increases, van der Waals' forces of attraction increases, hence boiling point increases.
(d) Boiling point decreases with branching because surface area decreases, van der Waals' forces of attraction decreases.
(e) Inflammability decrease with increase in number of halogen atoms e.g. CCl4 is used as fire extinguisher under the name pyrene. -
(a) It is because \(\mu\) = e x d, C-F bond distance is less than C-Cl, therefore, product of charge and distance is slightly lower in CH3F than CH3Cl.
(b) t is due to lower bond dissociation enthalpy of C-I bond than C-Cl bond.
(c) p-dichlorobenzene fits into crystal lattice more readily than o-isomer, therefore, has higher melting point. o-isomer has higher boiling point due to higher dipole moment, more polarity than p-isomer.
(d) \(\mu=\sqrt{\mu_{1}^{2}+\mu_{2}^{2}+2 \mu_{1} \mu_{2} \cos \theta}, \mu_{1}\) and \(\mu\)2 2 are dipole moments of C-X bond \(\mu_{1}=\mu_{2}\) and cos 60 = 0.5 in o-isomer cos 120 0 = -0.5 in m-isomer.
(e) It is due to C-I bond is longer than C-F bond and \(\mu\)= e x d.






































